Immobilization of β-Galactosidase on Monolithic Discs for the Production of Prebiotics Galacto-oligosaccharides
Abstract
Four different activated methacrylatic monoliths, acting as pore-through-flow membrane reactors in order to reduce enzyme costs and mass-transfer limitations, were characterized as support for immobilization of β-galactosidase to produce galacto-oligosaccharides (GOS). GOS synthesis was studied in different operation modes. A higher affinity of the immobilized enzyme towards the transgalactosylation reaction could be obtained, which results in desired GOS with higher degree of polymerisation. Kinetic models were successfully parametrized forming a basis for process modeling and optimization.
1 Introduction
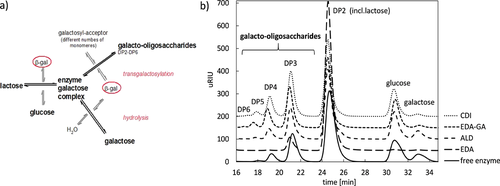
The growing health awareness of modern consumers generates an increasing demand of functional foods and food additives like prebiotics. In particular, galacto-oligosaccharides (GOS) enjoy an elevated interest, especially for their positive physiological properties on humans. They can be found in nature, e.g., as part of the human breast milk, and are considered to be responsible for the prebiotic effect 1. To cover the present demand, the development of efficient production strategies is essential. On industrial scale GOS are produced enzymatically from bovine lactose, a waste product of the dairy industry on a large scale 2 with the cost-intensive enzyme β-galactosidase (β-gal, EC 3.2.1.23) producing GOS in a side reaction (transgalactosylation), as shown in Fig. 1a. The product is GOS with different chain lengths, comprising 2–9 monomers (Fig. 1b) 3, expressed by the degree of polymerization (DP).
The application of enzymes in an immobilized form opens new possibilities and is widely used in biocatalysis and bioprocess technology. The advantages of immobilized enzymes compared to free enzymes are the increase in stability, the opportunity to operate continuously and the sustainability with respect to the consumption of cost-intensive enzyme is significantly improved. However, the selection of the support is crucial for the immobilization efficiency and mass transfer 4. One type of immobilization supports are monoliths, which are methacrylate-based macroporous carriers with suitable hydrodynamic properties and are used as convective interaction media (CIM®) 5-8. The methacrylate monoliths are characterized by high mechanical and chemical stability as well as an efficient mass transfer by convective pore-through-flow. In batch systems with free enzyme and in conventional particulate porous supports, the substrate has to diffuse into the pores to reach the active center of the immobilized enzyme, which determines the kinetics. On the contrary, the monolithic pore-through-flow reactor allows a convective dominating transport of the molecules in the pore structure to interact with the immobilized enzyme, so the transport of reactants and products is not limited by diffusion 6, 9, 10.
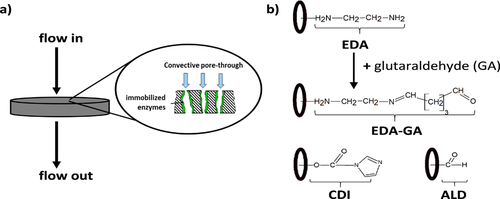
To reduce the protein surface interactions and to maintain a stable linkage with the monolith, the immobilization technique must be well selected 8. Several strategies can be used to couple β-gal on a CIM® monolithic pore-through-flow reactor, available with different chemistries 5-8. The immobilization by means of covalent bonds between the enzyme and the support was realized with a carbonyldiimodazole (CDI) monolith, an ethylenediamine (EDA), pre-activated with glutaraldehyde (GA), and an aldehyde (ALD) derivatization. The coupling via ionic interaction can be carried out by means of the EDA chemistry (weak anion exchanger without pre-activation with GA) of the CIM® monolith.
Aim of this contribution is the immobilization and characterization of β-gal on monolithic bioreactors to catalyze the GOS synthesis. Therefore, four different linkers of the methacrylate support in form of CIM® discs were studied experimentally and kinetic parameters for modeling were derived. To evaluate the potential of the immobilized enzymes, the results in particular regarding GOS yield in three different reactor operation modes were compared and evaluated to the batch reactor system with free enzyme.
2 Material and Methods
2.1 Chemicals and Analytics
A commercial β-galactosidase, originated from B. circulans, was used. Carbohydrate content was analyzed via HPLC and the protein content was determined by using a biuret-based reaction 11. More details are described in the Supporting Information (SI), Sect. S1.1.
2.2 Monolithic Enzyme Pore-through-flow Reactor and Enzyme Immobilization
CIM® CDI, EDA and ALD monoliths (Fig. 2), purchased from BIA Separations, were used as supports for β-gal immobilization. The methacrylate monoliths in form of discs (Fig. 2a), volume of 0.34 mL for CDI and EDA discs (pore size 1.3 µm) and of 0.1 mL for the ALD disc (pore size 2 µm), were installed in specialized housings. The immobilization procedure was carried out in accordance with BIA Separations 7 as described Sect. S1.2 (SI). All preparation steps were performed at a flow rate of 0.5 mL min−1, realized with a plunger pump (Knauer), at room temperature.
2.3 Experimental Configurations for the GOS Production
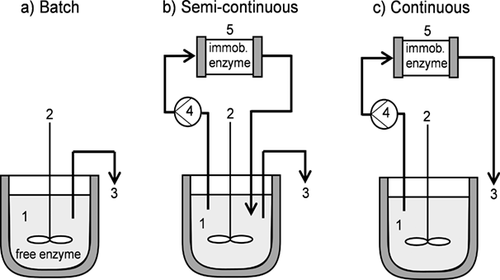
Three different reactor configurations for the GOS synthesis have been studied experimentally (Fig. 3): the conventional batch reactor (stirred tank) with free β-gal (Fig. 3a) as reference concept, the semi-continuous (Fig. 3b) and continuous systems (Fig. 3c) for the immobilized enzymes. The monolithic discs have been fed with substrate solution, which was pumped by means of a plunger pump (Knauer). A detailed description of the experimental procedure of the GOS synthesis with free and immobilized enzyme is provided in Sect. S1.3 (SI).
2.3.1 Determination of Performance and Kinetic Parameters
 ) is calculated according to Eq. 1:
) is calculated according to Eq. 1:
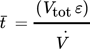 ()
()wherein Vtot is the total volume of the monolith, ε is the estimated porosity (ε = 0.3 for CDI and EDA discs, ε = 0.6 for ALD disc) and 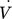 is the flow rate through the disc. The calculation is performed for one cycle (semi-continuous system), defined as the required time to circulate the whole volume of substrate solution through the monolith or for one module pass-through in case of the continuous configuration.
is the flow rate through the disc. The calculation is performed for one cycle (semi-continuous system), defined as the required time to circulate the whole volume of substrate solution through the monolith or for one module pass-through in case of the continuous configuration.
The specific activity (U mgenz−1) was calculated immediately after immobilization of β-gal for each monolithic support at a flow rate of 0.5 mL min−1 with the configuration setup shown in Fig. 3b and for long time experiments/storage up to 90 days after loading the monoliths. One unit is defined as the amount of enzyme that catalyzes the conversion of 1 µmol of lactose per minute in the liquid reactor volume (EDA, EDA-GA, CDI discs = 0.102 mL, ALD disc 0.06 mL, batch = 40 mL).
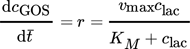 ()
()The maximal rate vmax (gGOSL−1min−1), residence time, and Michaelis-Menten constant KM (glacL−1) were determined with Lineweaver-Burk methods, where r is the reaction rate of GOS formation, c is the concentration of GOS or lactose (g L−1) and 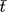 is the residence time of lactose in the reactor. Studies were performed at initial lactose concentrations (ILC) of 50, 100, 150, 200 and 220 g L−1.
is the residence time of lactose in the reactor. Studies were performed at initial lactose concentrations (ILC) of 50, 100, 150, 200 and 220 g L−1.
3 Results and Discussion
3.1 Kinetic Parameters and Evaluation of GOS Production with Immobilizedβ-gal
The analysis of produced GOS species with HPLC (Fig. 1b) reveals that it is possible to synthesize a higher amount of GOS – even with DP > 2 – with immobilized enzymes (semi-continuous system) than with free enzyme at the same lactose conversation level. Thereby, the EDA activated disc is an exception. The enzyme was supposed to bind via ionic interactions, but the preliminary experiments proved that most of the enzyme was washed out, so the amount and finally the activity of enzyme on disc was lower to produce GOS in a reasonable quantity. The amount of enzyme (Tab. 1) in the batch system comprised 9.02 mg in 40 mL reaction volume with a specific activity of 21.7 U mgenz−1. Shown as well in Tab. 1 is the theoretical possible amount of enzyme that might initially be bound to all the monolithic supports, which was determined by means of the protein assay only. As described in literature 4, 12, 13 the specific activity of immobilized enzymes is lower than the activity measured within the free enzyme system. The decrease in activity might be explained with steric hindrances and conformational effects after the enzyme binding on the support. Bertrand et al. 12 achieved a 40 times lower specific activity with an immobilized endodextranase on epoxy-activated discs and Tavernier et al. 13 characterized an activity that is even 1000 times lower for an immobilized glucuronan lyase also on an epoxy monolithic disc. For process modeling, design and optimization kinetic parameters for a Michaelis-Menten (Eq. 2) approach were estimated and summarized in Tab. 1.
|
β-gal |
menz [mg] |
Specific activitya) [U mgenz−1] |
KMb) [g L−1] |
vmaxc) [g L−1min−1] |
vmax,spec [g L−1 min−1 mgenz−1] |
Enzyme substrate ratiod) [U gsubst−1] |
|---|---|---|---|---|---|---|
|
free |
9.02 |
21.7 |
140 |
3 |
0.3 |
49 |
|
immob. on EDA disc |
3.41 |
0.3 |
N/A |
N/A |
N/A |
10 |
|
immob. on EDA-GA disc |
3.00 |
3.4 |
219 |
194 |
64.8 |
1020 |
|
immob. on CDI disc |
3.68 |
2.0 |
130 |
19 |
5.2 |
750 |
|
immob. on ALD disc |
0.50 |
4.0 |
171 |
6 |
11.2 |
323 |
- a), b), c) for immob. enzyme – semi-continuous system, flow = 0.5 mL min−1, b) glacL−1; c) gGOSL−1min−1; d) ratio between β-gal activity and lactose; N/A: not available data
The KM value for the immobilized enzymes on the CDI and ALD activated discs are comparable to the value for the free enzyme, which is due to the lack of mass transfer limitation of the substrate into the reactor pores. This is well described in literature 4. Using EDA-GA disc vmax was 65 times, with CDI 6 times and with ALD 2 times higher compared to the free enzyme (Tab. 1), which includes limitations by diffusion corresponding to observations made by 13. As well as seen before, the enzyme on the EDA-GA activated monolith shows different results. The explanation for the higher vmax and specific activity is the longer spacer arm between enzyme and support (Fig. 2b) that improves enzyme mobility, facilitates the interaction of substrate and enzyme and leads to a higher activity 14. These interesting results are more obvious using vmax,spec for EDA-GA, CDI or ALD. The β-gal on the EDA disc without pre-activation with glutaraldehyde obtains a very low binding performance to the support. Therefore, the determination of the kinetic parameters was not possible. It should be noted that for the immobilized enzyme the enzyme substrate ratio (U glac−1, Tab. 1) was significantly higher due to the much larger amount of bound β-gal in the active pore volume compared to the free enzyme system in 40-mL batch. This result of enzyme immobilization leads to higher reaction rates as reported in 8.
3.2 Performance of CDI, EDA-GA, EDA and ALD Activated Monoliths
The results of the final application of the GOS synthesis with free and immobilized β-gal are presented in Fig. 4. Depending on the enzyme-substrate contact time a higher GOS yield can be achieved with immobilized enzymes even with the use of significantly low enzyme concentrations. With free enzyme a yield of 23 % GOS was reached. This can be significantly increased by 17 % with the ALD, 52 % with EDA-GA activated discs and by 61 % with the β-gal on the CDI disc in a semi-continuous system (Fig. 4a). With the less amount of enzyme on the EDA disc (Tab. 1), the GOS yield could not be increased, it achieved 5 % only after 42 min. So far there are no results of GOS synthesis with enzyme immobilized on a methacrylate-based monolithic support activated with EDA-GA, CDI or ALD. Other supports like gelatine nanofiber mats led to an increase of GOS yield from 23 % with the free enzyme from A. oryzae to 38 % with the immobilized enzyme 15. Urrutia et al. 16 achieved same GOS yield with free and immobilized enzymes from B. circulans.
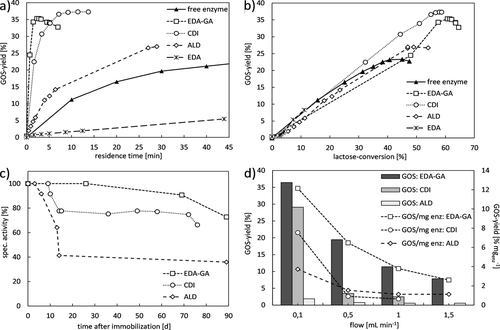
The enzyme on the CDI activated disc obtained the highest GOS yield and should therefore be used for further experiments. Thus, the dominating convective flow in the monolithic pores can efficiently reduce the influence of the diffusion limitation occurring in batch with the free enzyme. Another important observation is the higher affinity of the immobilized enzyme towards the desired transgalactosylation reaction than to hydrolysis (Figs. 1a and 4b) and improved selectivity. This is comparable with results obtained in 17. The GOS degradation starts at a lactose conversion rate of 55 % for the immobilized β-gal and at 40 % lactose conversion with the free enzyme (Fig. 4b), which leads to the formation of GOS with a higher, favorable DP using the monolithic disc. Thus, the hydrolysis as an undesired side reaction (Fig. 1a) is efficiently suppressed.
The dependence on flow rate is well described by Naldi et al. 14. Higher flow rates, resulting in a shorter residence time of lactose in the pore-through-flow reactor and reducing the GOS yield synthesized by the immobilized β-gal (Fig. 4d). The EDA-GA activation delivers contradicting results. Of all three activations, the EDA-GA delivers the best results for the continuous configuration, this is not the case with the semi-continuous configuration (Fig. 4a and Fig. 4d). The increased activity of the enzymes, bound to a longer and more flexible EDA-GA linker, leads to an increased yield in the continuous configuration where only the fresh substrate flows once through the monolith. In the case of the semi-continuous configuration, where in addition to the substrate lactose the by-products (GOS and monosaccharides) also flow through the reactor, this increased activity seems to lead to a faster degradation of the GOS.
The first study of long-term stability provided in Fig. 4c shows a slightly reduction in activity depending on the immobilization chemistry. The loss of activity for the EDA-GA disc was 27 %, for the CDI disc 34 % and the enzyme on the ALD disc resulted in 64 % activity loss 80 days after immobilization. 17 days after immobilization the EDA disc exhibits a hardly measurable activity (data not shown).
4 Conclusion
Four different chemical linkers were tested to immobilize β-gal: methacrylate monoliths activated with EDA-GA, EDA, CDI or ALD. The experiments demonstrate that the immobilized β-gal from B. circulans can be used for the synthesis of GOS by lactose derivatization. A significant increase in GOS yield and production of the prebiotics GOS with higher DP is possible. Thus, a shift of selectivity towards the desired transgalactosylation reaction takes place in comparison to the free enzyme system in a stirred tank. In addition, immobilized enzymes retain the activity over a long time and the enzyme substrate ratio is significantly increased. Consequently, enzyme costs can be drastically reduced. The enzyme cost reduction results in the reusable and/or continuous use of the enzyme over 80 days and the high enzyme substrate ratio for the immobilized reactor compared to the free enzyme system. The best results regarding maximum GOS yield and long-term stability could be achieved with β-gal immobilized on the CDI and EDA-GA activated discs, where the enzyme on the CDI disc revealed an increase in GOS yield of 61 % compared to the system with free enzyme. Consequently, these two linkers can be selected for prospective investigations towards scale-up and GOS synthesis in a recirculation system and for further process designs. The ALD support offered the highest activity loss of 64 % and the binding of β-gal via ionic interaction on the EDA activated disc turned out to be insufficient, so that these two activations appear unsuitable for a GOS synthesis from lactose with immobilized β-gal. Finally, kinetic models were parametrized forming the basis for process simulation, development and optimization.
Supporting Information
Supporting Information for this article can be found under DOI: https://doi.org/10.1002/cite.202000231. This section includes additional information for material and methods applied.
Acknowledgements
The authors gratefully acknowledge the funding by the German Federal Ministry of Education and Research (BMBF) within the framework of Research at Universities of Applied Sciences 2018 (project number: 13FH574IX6).
Furthermore, the authors would like to appreciate the project partner BIA Separations for the providing CIM® hardware, visual material and technical support. Open access funding enabled and organized by Projekt DEAL.
Symbols used
-
- c [g L−1]
-
concentration
-
- KM [g L−1]
-
Michaelis-Menten constant
-
- menz [mg]
-
mass of enzyme
-
- r [g L−1min−1]
-
reaction rate
-
- t [min]
-
experimental running) time
-
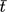 [min]
[min] -
residence time
-
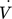 [mL min−1]
[mL min−1] -
flow rate
-
- vmax [g L−1min−1]
-
maximal velocity
-
- vmax,spec [U mgenz−1]
-
maximal specific velocity
-
- Vtot [mL]
-
total volume
-
- ε [–]
-
porosity
Subscripts
-
- enz
-
enzyme
-
- immob
-
immobilized
-
- lac
-
lactose
-
- tot
-
total
-
- spec
-
specific
-
- subst
-
substrate
Abbreviations
-
- B. circulans
-
Bacillus circulans
-
- β-gal
-
β-galactosidase
-
- CIM®
-
convective interaction media®
-
- CDI
-
carboxyimidazole
-
- DP
-
degree of polymerization
-
- EDA
-
ethylenediamine
-
- EDA-GA
-
ethylenediamine activated with glutaraldehyde
-
- GOS
-
galacto-oligosaccharides
-
- HPLC
-
high performance chromatography
-
- ILC
-
initial lactose concentration
-
- immob
-
immobilized
-
- U
-
units (enzyme activity)




