Self-Assembled Chiral Film Based on Melanin Polymers
This article is a contribution to the special issue “Proceedings from 33rd Symposium on Chirality in Rome, Italy 2023.”
ABSTRACT
Chirality plays a fundamental role in natural phenomena, yet its manifestation on solid surfaces remains relatively unexplored. In this study, we investigate the formation of chiroptical melanin-based self-assembled films on quartz substrates, leveraging mussel-inspired surface chemistry. Water-soluble porphyrins serve as molecular synthons, facilitating the spontaneous formation of hetero-aggregates in phosphate-buffered saline containing L- or D-DOPA. Spectroscopic analysis reveals chiral transfer from DOPA enantiomers to porphyrin hetero-aggregates, followed by the disruption of these latter and subsequent generation of chiral melanin structures in solution. Quartz substrates inserted into these solutions spontaneously accumulate homogeneous melanin-like films over days, demonstrating the feasibility of self-assembly. The resulting films exhibit characteristic UV/Vis and CD spectra, with distinct signals indicating successful chiral induction. Interestingly, the AFM characterizations reveal a distinct surface morphology, and in addition, some thermal and mechanical properties have been taken into account. Overall, this study sheds light on the formation, stability, and chiroptical properties of melanin-based films, paving the way for their application in various fields.
1 Introduction
Chiral nanostructures and materials have garnered significant attention in recent years, given their ability to exhibit distinctive properties and practical functions [1-3]. This has opened up numerous new opportunities for applications in asymmetric catalysis [1, 4], chiral recognition, separation [5-8], chiroptic devices [9-13], and so forth. Therefore, due to its significance, chiral processes have been widely investigated within three-dimensional systems, such as crystals or liquid solutions [14, 15]. However, at the same time, there is considerably less knowledge regarding chirality in relation to two-dimensional structures, particularly solid surfaces [16]. As a matter of fact, the comprehension of chirality on solid surfaces is emerging over time and numerous works have been focusing on elucidating the principles and methods for creating solid surfaces with distinct enantioselectivity [16-21]. Overall, given the small energy differences between chiral species (i.e., a few kilojoules per mole), both at a molecular and mesoscopic level, a surface becomes chiral only if a significant level of homogeneity and stereoregularity is reached on the solid surface. Thus, the first matter to solve is how to achieve a chiral surface. The first method involves the easy (covalently or not) adsorption of a chiral molecule onto an achiral surface. As a consequence, the adsorbed molecule breaks the symmetry of the surface, making it chiral [22-28].
Another method consists of realizing chiral self-assembled films onto various solid surfaces by the spontaneous chiral self-assembly of achiral (or chiral) molecules in a liquid solution [29-33]. Noteworthy, the adhesion of the final chiral film onto the solid surface is crucial for ensuring further applications of the functionalized substrate.
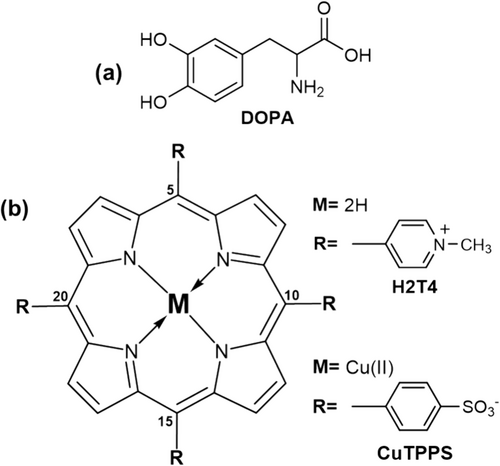
In this context, mussel-inspired surface chemistry based on melanin coatings can represent a valuable strategy [34-36]. Melanins, or eumelanins, are black insoluble polymers derived from the spontaneous oxidation, in an aqueous solution and under specific experimental conditions [37, 38], of catecholamine precursors, such as the (3,4-dihydroxyphenylalanine (DOPA, Figure 1a) [39, 40]. Although the exact DOPA polymerization mechanism is still under investigation [35, 40-42], numerous studies reported the use of catecholamines and their derivatives to realize adhesive film on various surfaces [43-46]. The final melanin polymer contains several moieties (e.g., carboxy, quinolone, hydroxyl, and amine groups) able to form an extensive network of interactions (hydrogen bonds, electrostatic and hydrophobic interactions, or plausible covalent couplings) with the substrate, which are responsible for the strong underwater adhesion [35]. It is worth mentioning that DOPA is an aromatic amino acid, and particularly its L-enantiomer is naturally expressed in mammalian species. As such, the L-DOPA is a chiral molecule and one may speculate that its subsequent spontaneous oxidative polymerization, in the presence of a certain substrate, could lead to the deposition of chiral melanin film. Analogously, an opposite chiral film could be obtained from the corresponding D-enantiomer. Unfortunately, the L- or D-DOPA oxidative polymerization proceeds with the loss of the chiral center, leading to the formation of achiral melanin polymers as well [40].
Nowadays, the knowledge about the chiroptical characteristics of melanin polymers is rather limited. A single study reports the possibility to bestow chirality to melanin polymers caused by atropisomerism of some catecholamine precursors [47]. Besides, a few papers report on chiroptical materials based on melanin polymers [48-50].
In this regard, recently we have demonstrated the formation of chiral melanin oligomers exploiting chiral porphyrin hetero-aggregates induced by L- or D-DOPA [40]. The experimental strategy started from chiral induction phenomenon from L- or D-DOPA to porphyrin hetero-aggregates, composed of meso-tetrakis (4-N-methylpyridyl) porphyrin (H2T4, Figure 1b) and copper (II) meso-tetrakis(4-sulfonatophenyl)porphyrin (CuTPPS Figure 1b), in an aqueous solution. In fact, as a result of extensive supramolecular interactions, porphyrin hetero-aggregates own kinetic inertness [51] to “store” the chiral information from chiral templates (e.g., DOPA enantiomers) by the “Sergeants-and-Soldiers” principle [52-54]. However, during the spontaneous oxidative conversion of DOPA enantiomers to melanin polymers, we unexpectedly observed the disruption of H2T4/CuTPPS porphyrin hetero-aggregates giving rise to the release of CuTPPS in the solution and H2T4 entrapping into the developing melanin matrix [40]. Interestingly, when the chirality of DOPA is progressively lost, the chiral porphyrin hetero-aggregate served in turn as a template (i.e., chiral feedback) for imprinting their chirality to developing melanin oligomers. This phenomenon represents a rare example of temporary chiral mediation [40].
Thus, by exploiting (i) the properties of melanin polymers to form stable and adhesive film on various solid surfaces, especially glass or quartz substrate [55], and (ii) the temporary chiral mediation by H2T4/CuTPPS porphyrin hetero-aggregates [40], herein, we report on the formation of chiroptical melanin-based self-assembled film on common quartz substrates, from the spontaneous oxidative polymerization of L- or D-DOPA in an aqueous solution.
2 Materials and Methods
3,4-L-dihydroxyphenylalanine (L-DOPA, C9H11NO4, MW = 197.19 g/mol, CAS number 59-92-7, purity > 98%) and the respective enantiomer, 3,4-D-dihydroxyphenylalanine (D-DOPA, C9H11NO4, MW = 197.19 g/mol, CAS number 5796-17-8, purity > 95%) were purchased from Merck company and used as received. Work solutions of L- or D-DOPA were freshly prepared by dissolving the appropriate amount of solid in PBS buffer to achieve a concentration of 0.5 mM.
The phosphate-buffered saline (PBS) was prepared by solubilizing one tablet, purchased from Merck company (product number: P4417) in 200 mL of ultrapure water. The resulting buffer (pH = 7.4) contains 10 mM of sodium phosphate ([H2PO4−] + [HPO42−] = 10 mM), 137 mM of sodium chloride, and 2.7 mM of potassium chloride.
Ultrapure water at room temperature (18.2 MΩ cm, TOC 1 ppb) obtained from the PURELAB Flex 3 system (Elga Veolia company) has been used to prepare all samples and stock solutions.
The 5,10,15,20-tetrakis(4-N-methylpyridyl)porphyrintetrachloride salt (H2T4, C44H38Cl4N8, MW = 820.64 g/mol, CAS number 92739-63-4, purity > 95%) was purchased from the Mid-Century company and used as received.
The Cu(II) derivative of 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin (CuTPPS) was synthesized from the corresponding copper(II) oxide (CuO) by heterogeneous metal-insertion of 5,10,15,20-tetrakis-(4-sulfonatophenyl)porphyrin tetrasodium salt (TPPS, C44H26N4Na4O12S4, MW = 1022.91 g/mol, CAS number 39050-26-5, purity > 95%, from Mid-Century company) in ultrapure water, as previously reported [56].
All stock porphyrin solutions (about 4 × 10−4 M, stored in the dark at room temperature) were prepared by dissolving the proper amount of the solid in ultrapure water. The concentration of all stock solutions was spectrophotometrically checked in aqueous solution, at neutral pH, by means of the corresponding molar extinction coefficients at the maximum of the Soret band: λmax(H2O) = 423 nm (ε = 224,000 M−1 cm−1) for H2T4 and λmax(H2O) = 412 nm (ε = 416,000 M−1 cm−1) for CuTPPS.
The porphyrin hetero-aggregates in PBS buffer were obtained by adding the proper volume (to reach a 2 μM concentration) of H2T4 stock solution to the sample solution. After 5 min, the amount (to reach again a 2 μM concentration) of CuTPPS was added to the sample solution. After an additional 20 min, other aliquots of H2T4 and CuTPPS were added as illustrated before. The final sample solution, thus obtained, was kept for 20 min before spectroscopic investigations.
The porphyrin hetero-aggregates in the presence of D- or L-DOPA were obtained by using the corresponding DOPA solution (0.5 mM in PBS) following the aforementioned procedure. In detail, the proper volume of H2T4 stock solution was added to D- or L-DOPA solution to reach a 2 μM concentration of H2T4, and then after 5 min, the proper amount of CuTPPS was added to the sample solution in order to reach again a 2 μM concentration of CuTPPS. After an additional 20 min, other aliquots of H2T4 and CuTPPS were added as illustrated before. The final sample solution, thus obtained, was kept for 20 min before spectroscopic investigations. For the long incubation time, each solution of DOPA and porphyrin hetero-aggregates was stored in sealed plastic vials to limit the adhesion of both porphyrins and DOPA on the walls.
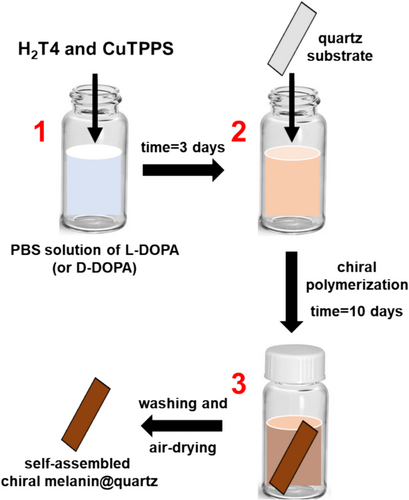
For the deposition experiment, quartz substrates (thickness = 1 mm), purchased from Helios Quartz company, have been properly cut in a rectangular form (≈ 0.6 cm × 2.5 cm, surface area ≈ 1.5 cm2). Each substrate was thus washed with (i) an acid hydroalcoholic solution (1% v/v conc. HCl, 70% v/v ethanol), (ii) ultrapure water, and (iii) dried under a nitrogen atmosphere.
Then, the as-prepared quartz substrate was inserted into 3 days old PBS solution containing L-DOPA (or D-DOPA) and porphyrin hetero-aggregates (Figure 2, second step). After 2 weeks of incubation (Figure 2, third step), the functionalized quartz substrate was removed from the solution, washed with ultrapure water, and dried in air. Moreover, in order to assess the reproducibility of the deposition experiment, each sample was prepared at least three times by using independent porphyrins stock solutions, DOPA work solutions, and new quartz substrates.
All experimental procedures were performed in ultraclean conditions: (i) the operators wore a lab coat, hair cap, gloves, and mask during the preparation of samples and (ii) the tips of the pipettes and the plastic cells were washed three times with ultrapure water before being used.
A JASCO V-530 UV/Vis spectrophotometer was used for the spectroscopic investigations at room temperature. Circular dichroism (CD) measurements were carried out at room temperature on a JASCO J-810 spectropolarimeter. In both cases, a quartz cuvette 1 cm path-length and an appropriate solid sample holder were employed for investigating liquid samples and quartz substrates, respectively. For the quartz substrate characterizations, the CD spectra were also collected from two opposite faces at 0° and rotated by 90° and 180°.
For the thermal stability tests, a Osram Ultra Vitalux 300 W E27 lamp was used. The evaluation of the mechanical properties was performed by scratch experiments on melanin@quartz substrates, that is, delayering the upper layers with a common scotch tape.
Morphological characterization and roughness measurements were performed by Atomic Force Microscopy (AFM). The instrument employed was a NT-MDT modular AFM NTEGRA instrument equipped with a PX Ultra controller system. The measurements were performed in semicontact mode and an equipped tip of high-resolution silicon tip (ETALON HA_NC type, resonant frequency 140 kHz ± 10% kHz, force constant 3.5 N/m ± 20% N/m, cantilever length 124 μm ± 2 μm, cantilever width 34 μm ± 3 μm, cantilever thickness 1.85 μm ± 0.15 μm). The measurements were performed at atmospheric conditions. The root mean square (RMS) and roughness average (RA) were chosen as fundamental parameters of samples' roughness estimation. Additionally, the peak-to-peak (PtP, maximum height), the developed surface area ratio (Sdr, the ratio between the real sample area over the nominal scan area), and the average lateral distance between peaks of a section (Sm) were considered as others parameters to study differences between the two melanin films. The acquisition scan area was chosen as 4 μm2. A total of three different scans were performed on different zones of each sample to acquire measurements of statistical evidence.
3 Results and Discussions
Water-soluble porphyrins serve as a paradigm of molecular synthons for creating functional self-assembled materials in an aqueous solution. Initially driven by electrostatic interactions, in water, oppositely charged porphyrins spontaneously give rise to hetero-aggregates. The process is hierarchically governed, and the final aggregates display exceptional stability and inertness. The latter allows for the memorization of the chiral information in the presence of any chiral physical (e.g., vortices, hydrodynamic flows, magnetic fields, and temperature gradient) or chemical (e.g., contaminants and templates) perturbation [8, 40, 57-62]. This happens when an equimolar amount (4 μM) of cationic H2T4 and anionic CuTPPS are added to a PBS solution containing 0.5 mM of L-DOPA or D-DOPA. The chiral information is transferred from the template to the hetero-aggregates as denoted by the specular exciton coupling in the visible region of induced circular dichroism (ICD) spectra (Figure 3). Noteworthy, the exciton coupling is due to the strong electric dipole–dipole coupling between the porphyrin monomers, which is, in turn, responsible for the bisignate CD couplet [63]. Therefore, slight structural changes in the resulting supramolecular hetero-aggregates can affect the final dichroic response yielding not ideal specular signals (Figure 3, solid lines). In the ultraviolet region, about 280 nm, it is noted the positive and negative dichroic signals related to L-DOPA and D-DOPA, respectively (see also Figure S1). The construction of the H2T4/CuTPPS hetero-aggregates is also denoted by the hypochromic effect and broadening of the Soret bands in the visible region (λ ≈ 420 nm) of UV/Vis spectra (Figure S2). On the other hand, if the hetero-aggregates are formed in the absence of any chiral perturbations, no optical activity is clearly revealed in all wavelength range of the CD spectrum (Figure S3). The successful formation of such porphyrin hetero-aggregates represents the first step of our experimental strategy (Figure 2) to obtain chiral films based on melanin-polymers. Then, we incubated the two solutions (one for each DOPA enantiomer) for 3 days in sealed plastic vials. In this period, both solutions become dark due to the oxidation of L-DOPA (or D-DOPA). This phenomenon is followed by some characteristic spectroscopic features (Figure 3): (i) a decrease and evolution of the CD signal related to DOPA enantiomers at 280 nm and (ii) nearly disappearance of the induced dichroic signal associated to porphyrin-hetero-aggregates. Similar changes are observed in the UV/Vis spectra of the incubated solutions (Figure S2). On the one hand, we observed the reduction of the UV/Vis signal of the DOPA enantiomers due to their polymerization in the solution (Figure S2); on the other hand, we detected the loss of the hetero-aggregate spectroscopic signal followed by the release of the CuTPPS porphyrin in the PBS solution, as highlighted by the appearance of the band at 412 nm, the latter more evident from the second derivative UV/Vis spectra (Figure S2-inset).
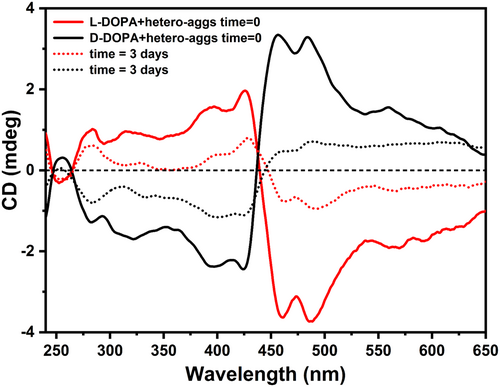
The cationic H2T4 porphyrin remains embedded in melanin polymers, whose surface is negatively charged in our experimental conditions, confirming the disruption of the porphyrin hetero-aggregates [40, 55]. However, undetectable chiral seeds of the hetero-aggregates [51] remain in solution driving the generation of new asymmetric melanin structures as evidenced by the evolution of the CD signal after 3 days (Figure 3) [40].
In this stage, we decided to insert a quartz substrate in the solution (Figure 2, second step). Knowing the adhesive properties of the melanin-polymers [34, 35, 43-46, 55], we supposed that as soon as chiral oligomeric melanin structures are formed in the solution, they could stick onto the surface of the quartz substrate giving rise a self-assembled chiral film.
Hence, the quartz substrate was dipped in the PBS solution containing L- (or D-) DOPA and kept for 10 days. We observed, over time, a spontaneous deposition of a homogeneous melanin-like film on the quartz surface. It is worth mentioning that a similar deposition time was previously employed to get the best coating rate and surface homogeneity [55].
Afterward, the functionalized quartz substrate was gently washed with ultrapure water and air-dried to obtain the final self-assembled L- (or D-) DOPA-melanin film@quartz (Figure 2, last step and Figure 4a).
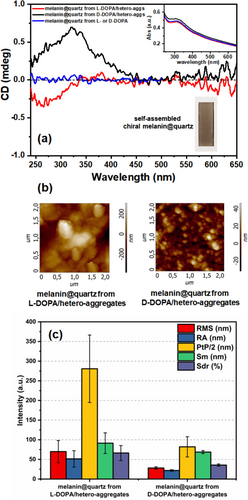
The UV/Vis spectra of both solutions, after the coating process, display the Soret band of the CuTPPS and just a little Rayleigh light-scattering (Figure S4). Despite the undeniable presence of melanin particles in suspension, most of melanin-polymers are self-assembled onto the surface, and consequently, the solution is almost transparent. On the contrary, the UV/Vis spectrum of the functionalized quartz substrate reveals the typical melanins' continuum extinction [55] in the visible region except, for a broad band in the near UV region around 320 nm, plausibly owing to oxidized DOPA residuals and melanin oligomers embedded into the film (Figure 4a-inset). Moreover, no spectroscopic differences have been noted for the film prepared from opposite DOPA enantiomers, alone (as a reference) or in the presence of porphyrin hetero-aggregates.
Interestingly, the CD spectrum of the film obtained from the L-DOPA shows a clear-cut signal in the near UV region about 300 nm. A quasi-mirror image spectrum is obtained from the asymmetric polymerization of the D-DOPA (Figure 4a). As the film spectra were collected under different orientations (Figure S5), we can reasonably exclude linear dichroism (see also Section 2). In addition, the dichroic signal of DOPA-melanin film@quartz achieved from L- or D-DOPA alone in the solution is flat (Figure 4a, blue curve), ruling out the emergence of chiroptical activity due to chiral inherent effects during the surface covering. Analogously, the introduction of the quartz substrate as soon as the L- or D-DOPA/hetero-aggregates solution has been prepared (i.e., time = 0) leads to the deposition of not-chiral melanin films (Figure S6). This behavior may be due to the significant absence of sticking asymmetric melanin oligomers at the initial stages. Noteworthy, if the initial solution (Figure 2, first step) is incubated for more than 3 days (i.e., time = 7 days), we do not observe a significant formation of melanin films, because the over-oxidation of the catechol groups of DOPA; in fact, the hydrogen bondings between the catechol groups and the O atoms/OH moieties of the silicon dioxide substrates are firstly responsible for the film adhesion [64].
Therefore, according to the procedure reported in Figure 2, CD results confirmed the successful achievement of chiral melanin-based films (Figure 4a). CD spectra of chiral melanin films obtained from independent experiments (Figure S7) displayed identical results with respect to those reported in Figure 4a, confirming the reproducibility of the deposition as well.
Morphological characterizations on the chiral films were performed to further support our findings. In particular, such morphological evaluation of the two melanin systems was performed by AFM characterization. The surface morphology of melanin film from L-DOPA/hetero-aggregates is transversally different compared to film from D-DOPA/hetero-aggregates. The former film is characterized by micrometric spheres of melanin agglomerated with each other, forming high-level structures of peaks and valleys (Figure 4b). Conversely, the latter film is distinguished by micrometric spheres unable to form a complex surface texture of height differences (Figure 4b). The roughness fundamental parameters (RMS, RA, and PtP) were essential in evaluating the differences between the two melanin films derived by L-DOPA/hetero-aggs and D-DOPA/hetero-aggs. The melanin film from L-DOPA/hetero-aggs differs to that from D-DOPA/hetero-aggs for the higher roughness parameters values, as shown in Figure 4c. Specifically, the RMS and RA values of film from L-DOPA/hetero-aggs (respectively 69.9 nm ± 28.2 nm and 51.4 nm ± 20.8 nm) are slightly higher than the film from D-DOPA/hetero-aggs (respectively 28.2 nm ± 3.1 nm and 21.8 nm ± 2.1 nm). The more complex texture of peaks and valleys of the former film is numerically made explicit also by its decisive higher PtP value of than melanin film from D-DOPA/hetero-aggs system with values of 561.2% ± 171.7% and 164% ± 51.5%, respectively.
The PtP values in Figure 4 were divided by two to easily visualize the histogram graph. It has been found that the lateral AFM parameter (Sm) of film from L-DOPA/hetero-aggs is higher than that one from D-DOPA/hetero-aggs due to the complex surface texture of the first one. Lastly, the developed surface area (Sdr parameter) of film from L-DOPA/hetero-aggs is 66.3% ± 19% in contrast with the 35.6% ± 2.9% of film from D-DOPA/hetero-aggs and an average difference of 54% of developed surface area between the two samples. Although morphological characterizations of two enantiomeric melanin films are not extensively investigated yet in literature, it has been recently reported [50] that films from different enantiomers vary in porosity and surface that the oxidative polymerization of enantiomeric melanin-precursors can lead to morphological divergences.
Finally, we assessed the thermal and mechanical stability of our chiral melanin@quartz substrates (Figures S8–S10). The thermal treatment of melanin@quartz from L-DOPA/hetero-aggregates was conducted at 60°C for 15 min via solar lamp irradiation with the sample kept under ultrapure water immersion. After the thermal treatment, the substrate was investigated by circular dichroism measurements and morphological characterizations (Figure S8). Throughout the thermal test, no significant leakage of melanin film from the samples was observed. However, the CD spectrum reveals a limited loss of the chiroptical response (Figure S8a). The surface morphology remained similar (Figure S8b) with melanin sphere agglomeration as seen for the pristine samples (Figure 4b). The roughness and lateral size features are resumed in the bar charts of Figure S10. The thermal treated sample revealed RMS and RA roughness values of 27.16 nm ± 3.84 nm and 19.78 nm ± 1.97 nm, respectively. The developed surface area (Sdr) of the thermally treated sample is 17.99% ± 5.30%. As compared with the pristine melanin@quartz from the L-DOPA/hetero-aggregates sample, the roughness parameters were subjected to a decrement after the thermal treatment. Overall, the lower AFM parameters along with the reduced chiroptical properties may be attributed to a partial melanin surface rearrangement under a thermal treatment.
On the other hand, the mechanical stability of our samples was assessed by scratch experiments. The melanin film is not subjected to complete removal from the quartz substrate, pointing out good mechanical stability (Figure S9a, inset). This is also confirmed by AFM analysis that shows the presence of melanin on the quartz surface (Figure S9b). However, the chirality of the film is totally lost as confirmed by the corresponding CD spectrum (Figure S9a). The AFM parameters (Figure S10) displayed a more significant value decrement in comparison with the pristine sample (Figure 4b). In particular, RMS and RA roughness values are 17.00 nm ± 3.14 nm and 13.75 nm ± 2.43 nm, respectively. The Sdr parameter is 7.95% ± 0.57%. Thus, the scratch tests revealed different morphology and roughness which are reasonably reflected in the complete loss of the chiral properties.
4 Conclusion
In the present work, we illustrated a supramolecular strategy to obtain homogeneous chiral self-assembled films on a quartz substrate. The strategy involves a hierarchical process where the chiral information of the DOPA enantiomers is firstly transferred to porphyrin hetero-aggregates. During the oxidative DOPA polymerization to melanins, the temporary chiral mediation of the porphyrin hetero-aggregates drives the formation of asymmetric melanin structure over time. As the adhesive properties of melanins, in the presence of a quartz substrate, these asymmetric structures can cover the quartz surface resulting in a self-assembled film. Spectroscopic analysis confirmed the successful formation of the chiral film, and AFM characterizations also revealed distinct surface morphologies for films from different DOPA enantiomers. Besides, the thermal treatment partially alters the surface morphology and the chiroptical response of the melanin film. Conversely, mechanical stresses lead to a complete loss of the chiral response along with a different melanin surface morphology.
To date, such an approach represents the first example of generating chiral film based on melanins. The latter may pave the way towards a plethora of applications in mussel-inspired surface chemistry for chiral recognition and separation science and new chiroptic devices. For instance, chiral melanin films could exhibit a specific chiral recognition ability aimed at resolving racemic mixtures of chiral compounds. Chiral melanin films could also be used to create circular polarizers, which can selectively filter circularly polarized light. This is valuable in areas like optical communication and liquid crystal display technologies [65, 66]. Finally, the chiral nature of melanin films could enhance the sensitivity and selectivity of biosensors. In fact, the interaction of these films with biomolecules can be used to detect chirality-specific interactions, which are crucial in the detection of certain biomolecules or drugs [67, 68].
Author Contributions
Massimiliano Gaeta: conceptualization, formal analysis, investigation, visualization, writing–original draft, data curation. Gabriele Travagliante: investigation, validation, formal analysis. Matteo Barcellona: investigation. Maria Elena Fragalà: supervision. Roberto Purrello: methodology. Alessandro D'Urso: conceptualization, funding acquisition, methodology, project administration, supervision, resources.
Acknowledgments
This work was financially supported by Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) PRIN 2022 2022R9WCZS and PRIN 2022 PNRR P2022KY45H. Open access publishing facilitated by Universita degli Studi di Catania, as part of the Wiley - CRUI-CARE agreement.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




