Ice Crystal Growth in Sucrose Solutions Containing Kappa- and Iota-Carrageenans
Abstract
Measurements of ice recrystallization inhibition (IRI) and thermal hysteresis (TH) activities of kappa (κ)- and iota (ι)-carrageenans were carried out to examine whether they can be novel cryoprotectants or not. IRI measurements indicate that both carrageenans reduce recrystallization in sucrose solution, but that the IRI activity of κ-carregeenan is higher than that of ι-carrageenan. TH measurements indicate that κ- and ι-carrageenans do not exhibit TH activity. TH activity measurements of antifreeze glycoprotein (AFGP) in the presence of κ-carregeenan demonstrate that this carregeenan neither influences the TH activity of AFGP nor the shape of the ice crystals. The round ice crystal shape transformed into an angular and elongated shape in the presence of both carregeenans.
1 Introduction
Freezing is one of the most preferred methods not only to preserve sensible food ingredients but also to store, e.g., various types of cells, tissues, and organs. The quality of frozen food and the prevention of the deterioration of cells and organs are largely dependent on the freezing velocity and the temperature history during transportation and storage. The higher the temperature and the greater the temperature fluctuations, the faster are the deterioration processes like recrystallization. As a result of recrystallization, the size, number, and shape of the ice crystals are changing. Hence, while recrystallization affects the appearance and texture of frozen food, it results in lethal damage to cells and tissues.
Damage due to ice recrystallization is referred to as cryoinjury 1, 2 and can be inhibited by cryoprotectants 3. The most commonly used cryoprotectants are dimethyl sulfoxide (DMSO), glycerol, 1,2-propanediol, and various polymers such as hydroxyethyl starch and poly(vinylpyrrolidone) 4. The application of cryoprotectants, however, has some drawbacks, such as limited efficiency, requiring high concentrations, which can be toxic for cells and tissues, and influencing metabolic functions 3. Thus, the development of other naturally occurring substances like antifreeze proteins (AFPs) and hydrocolloids has been preferred for recrystallization inhibition.
AFPs are also called ice-binding proteins (IBPs) 5 because of their binding to ice crystal surfaces, thereby controlling crystal growth and recrystallization. AFPs may exert two forms of activity: ice recrystallization inhibition (IRI) activity and thermal hysteresis (TH) activity 6, 7. In the literature, there are lots of studies of the IRI activity of AFPs. It has been suggested that AFPs adsorb to ice surfaces and inhibit the growth of ice crystals. This adsorption results in depression on the freezing point of ice crystals and creates a gap between the melting point (Tm)1) and the non-equilibrium freezing point (Tf), which is known as thermal hysteresis (TH). The TH activity of AFPs has been assessed in terms of their influence on the morphology of ice crystals. Ice crystal morphology is modified by AFPs based on their binding to specific ice crystal planes, which results in particular ice shapes. For instance, while the ice crystals grow in the shape of lemons or ellipsoids of revolution in the presence of the hyperactive TmAFP from Tenebrio molitor 8, bipyramidal shapes with elongated c-axes have been observed in the presence of moderately active fish AFPs type I, II, and III 9.
The influence of moderate and hyperactive AFPs on the shape of ice crystals has been examined to reveal whether they can be considered as cryoprotectants or not. The results indicate that the uncontrolled growth of ice crystals in the form of spicules at temperatures below Tf in the presence of moderately active AFPs results in an increase in cryoinjury while hyperactive AFPs can be cryoprotectants, as sharp ice crystal shapes are not formed 10. Accordingly, despite the high IRI activity of AFPs, not all AFPs are good cryoprotectants. Additionally, AFPs are generally rarely used for industrial application because of their high price and the limited availability. Thus, alternative substances, which have a high IRI activity and high process stability are needed.
If a substance exhibits a high IRI activity but no TH activity, it has the properties needed for being used as an effective cryoprotectant. As indicated in the literature, various kinds of stabilizers are employed to produce ice cream with desired properties, which have an influence on phase separation, crystallization, recrystallization, etc. 11. One of the preferred stabilizers is carrageenan 11, which is mainly classified in three types: κ-carrageenan, ι-carrageenan, and λ-carrageenan. They differ from each other in their chemical structures, which cause differences in the hydration properties, strength, texture, and temperature of gel formation 12.
The hydrocolloid κ-carrageenan is of great importance as an IRI-active molecule 13-15. According to Gaukel et al., the hydrocolloid κ-carrageenan has a similar effect as AFPs on recrystallization 13. However, the mechanism of the inhibition effect of these hydrocolloids is not fully understood. Leiter et al. investigated the influence of several ions on the IRI activity of κ-carrageenan. They found that the IRI activity of κ-carrageenan strictly depends on the type of ion, and this dependence was explained by the relation between the presence of ions and gelation of κ-carrageenan. They concluded that this gelation caused the reduction of the interaction between the κ-carrageenan molecules with the ice crystal surface and, thus, the IRI activity of κ-carrageenan decreased in the presence of ions 14.
Even though κ-carrageenan has a high IRI activity, there is no investigation of the IRI activity of other types of carrageenans in the literature. Therefore, the IRI activities of κ- and ι-carrageenans in sucrose solutions were measured to reveal whether there is an influence of the types of carrageenan on the recrystallization or not. It is obvious that if κ-carrageenan binds to the ice crystal surface there should be a measurable TH activity. Thus, TH activities of κ-carrageenan and ι-carrageenan were determined in sucrose solution in this study. These measurements have been performed in sucrose solution firstly to have IRI activity results for κ-carrageenan comparable with the literature 13. Secondly, as indicated in this literature, especially in food applications, there are many ingredients such as sucrose in the product, which can affect the investigated effects.
2 Materials and Methods
2.1 IRI Activity Measurement
The IRI activities of κ- and ι-carrageenans were measured in 49 wt % sucrose solution using a method similar to the one described previously by Gaukel et al. 13. κ-Carrageenan extracted from the red seaweed Eucheuma cottonii and ι-carrageenan were purchased from Eurogum A/S (Herlev, Denmark). The sample solutions were prepared with a concentration of 1 mg mL−1 carrageenan one day before the experiments. Solutions of κ- and ι-carrageenans were made with demineralized water on a magnetic stirrer at 60 °C for 30 min until the carrageenans were dissolved completely. Afterwards, this solution was cooled down to room temperature and stored in a freezer. Before the experiment, the solution was melted at room temperature.
A specific amount (10 µL) of sample solution was placed between two microscope cover slips glued on an objective slide. The sample was covered with another cover slip and sealed with silicone. After drying of the silicone, samples were immersed in liquid nitrogen for subjecting them to fast freezing for a few seconds. In this way, the aqueous solution was transformed into a glassy state. These samples were stored at a constant temperature of −8.0 °C ± 0.01 °C in a storage chamber, which was placed inside a cooled glove box with a temperature of −10.0 °C ± 1 °C. An external cryostat (Model F30, Julabo GmbH, Seelbach, Germany) was used to keep the temperature constant in the storage chamber and on the microscope plate. The inside temperatures of both glove box and storage chamber and the temperature of the microscope plate were recorded by the thermocouples during the storage time of one week. Under these conditions, the ice volume fraction is approximately 10 % 16.
The photos of the ice crystals were taken by a digital camera (Altra SIS20, Olympus, Tokio, Japan) attached to a polarization microscope (BX41, Olympus, Tokio, Japan) placed in the glove box at certain time intervals (5 h, 27 h, 48 h, 99 h after freezing). The evaluation of the photos was made by the software ImagePro Insight 9.1 (Media Cybernetics, Rockville, USA). The contour of each ice crystal was manually circumscribed by a polygon tool and from the so-defined area of each crystal, the equivalent diameter was calculated as the diameter of a circle with the same area. The shape parameter circularity was calculated to identify the shape of the ice crystals (Circ= 4*Area/(π*MaxFeret2). For the evaluation, a minimum of 300 ice crystals were analyzed from each slide. The mean equivalent diameter of the number distribution was determined as x1,0 from these values. Each experiment was performed two times.
2.2 TH activity Measurement
TH activities were measured in 40 % (w/v) sucrose solution. In preliminary experiments performed in sucrose solution without additive it was found that this sucrose concentration is the optimal one at which single crystals are obtained more easily. The experimental setup consisted of a polarization microscope (Nikon Eclipse LV100ND) equipped with a digital camera (Nikon DS-Ri1) and a cold stage (Linkam LTS420). The cold stage was equipped with a microscope sample slide holder (G16M) with a stainless-steel ring on a pure silver heating/cooling block. The temperature gradient between the sample and the silver block was minimized by using a thin glass cover slip (16 mm in diameter, thickness of 0.13–0.17 mm) inside as basis for the sample solution. Two shim spacers (outer diameter OD 14 mm, inner diameter ID 12 mm × 70 µm) were placed on the glass cover slip to provide a gap for the sample solution and enough space for an ice crystal to grow. The sample of 0.5 μL was placed in 1 μl of immersion oil dropped in the middle of the cover slip to ensure uniform heat dispersion around the sample.
Besides the uniform sample temperature, the small amount of sample provides the advantage of having only one single crystal during crystal preparation. A small portion of immersion oil, which has the same refractive index as the glass cover slip, was used below the cover slip to provide a thermal seal between the glass and the stage. The upper surface of the sample was closed with another glass cover slip. After completing the sample preparation, the temperature of the stage was decreased to −30 °C to freeze the sample. Afterwards, the sample was heated to a temperature close to the melting point to obtain a single ice crystal. The crystal was then kept 10 min under these conditions and then cooled at a rate of 0.1 °C min−1 to detect the temperature at which the crystal grew suddenly. This sudden growth is referred to as bursting of the crystal. The temperature at this point is the non-equilibrium freezing temperature (Tf). Afterwards, this crystal was melted at the same rate to detect the melting temperature (Tm). The difference between these temperatures was calculated to determine the TH activity. Each experiment was repeated at least three times. The images were captured at a rate of 25 frames s−1.
The whole procedure was slightly adopted from Bar et al. 17 to meet the circumstances of the different equipment used in this study. TH activities of type I antifreeze protein (AFP I) and antifreeze glycoprotein (AFGP) were determined for checking the system for its convenience for TH measurements because it is known that the measurement technique has a significant influence on the TH determination. For instance, the TH activity of the beetle-derived AFP was measured at an approximately four times higher level via the nanoliter osmometer than when using the capillary technique 18. Therefore, preliminary experiments have been carried out to reveal whether TH activities of AFP I and AFGP can be detected with this system.
AFP I is a low-molecular-weight protein (< 30 kDa) obtained from the barfin plaice (bp), also called the native bpAFP 19 with a molecular weight of 3.5 kDa 20. AFGP was provided by Nichirei Co. Ltd. as a purified sample of AFGP from Eleginus gracilis 21 with an assumed molecular weight of 15 kDa 20. The TH activity of AFP I was stated in 0.1 M ammonium bicarbonate (pH 7.9) at a concentration of 10 mg mL−1. The value was found to be approximately the same as the one reported in the literature. AFP I showed a TH of 0.82 °C. This value was determined to be approximately 0.8 °C at the same concentration with the same type of AFP I (native bpAFP) in 0.1 M ammonium bicarbonate (pH 7.9) 19. According to these results, it can be concluded that this system is suitable for measuring TH activity.
The growth of an ice crystal in the presence of 5 mg mL−1 AFGP in 40 % sucrose solution is illustrated in Fig. 1. As expected, the ice crystal grows along the c-axis with time. This modified shape is attributed to binding of AFGP on the ice crystal. Fig. 1a displays the six prism planes of a single ice crystal grown in the presence of AFGP in 40 % sucrose solution. When the temperature is decreased further, AFGP binds to the specific planes of the single ice crystal; new disks join continuously and the ice crystal grows along the c-axis. The hexagonal bipyramidal shape is observed at the end of this process (Figs. 1b and c). This shape modification is in accordance with what is described in the literature. The same shape modification was observed in the presence of various types of AFGP, e.g., in the presence of the smallest AFGP (native AFGP8) 22 even though these measurements were performed in buffer solutions with different measurement techniques.
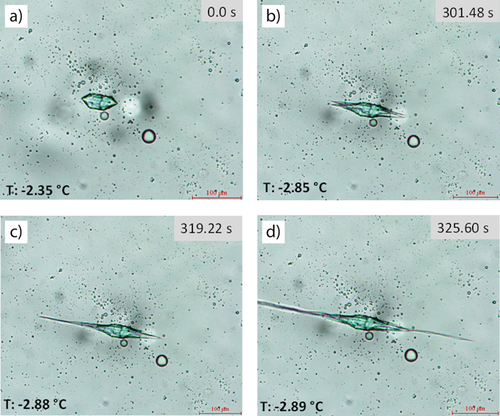
Fig. 1d illustrates the so-called burst growth where the temperature is detected as non-equilibrium freezing temperature. The TH value was calculated as 1.45 °C with 0.02 °C standard deviation. However, the TH activity values of different types of AFGP described in the literature are lower than those obtained in this study. These differences may be attributed to various reasons: annealing time, cooling rate, measurement technique, solution content, etc. For instance, in the literature, the TH activity of AFGP purchased from the same company, Nichirei Co. Ltd., is described to have been measured using a different technique, i.e., the capillary technique and in 0.1 M ammonium bicarbonate (pH 7.9). The results are lower than the results obtained in this study 20.
As seen in Fig. 1, the unique ice crystal morphology is observed as a result of the interaction of ice with a substantial number of AFGP. Peltier et al. revealed that there is a significant relationship between strong shape modification and TH. They synthesized some analogues of the smallest AFGP (AFGP8) and measured their TH activity while observing their shape modifications. When they detected the same strong shape modification in the presence of 80 mg mL−1 of analogues 12 and 13 as seen in Fig. 1, they determined the TH values to be 0.14 °C ± 0.1 °C and 0.15 °C ± 0.07 °C, respectively. However, one of the analogues (analogue 11) of synthesized AFGP8 did not exhibit faceting at lower concentrations than 80 mg mL−1. Even at 80 mg mL−1, the irregular hexagonal plate shape was observed and they did not detect TH activity for this analogue 22.
3 Results and Discussion
3.1 Influence of Various Types of Carrageenans on Recrystallization Inhibition
IRI increases with the addition of κ- and ι-carrageenans to the basic sucrose solution. The results of κ-carrageenan are conform with those described Gaukel et al. 13. The IRI activity measurements of ι-carrageenan relative to the IRI activity of κ-carrageenan are presented in Fig. 2 and Tab. 1.
|
Sample |
Storage time [h] |
|||
|---|---|---|---|---|
|
5 |
27 |
50 |
99 |
|
|
49 % sucrose solution |
||||
|
Mean ice crystal diameter x1,0 [μm] |
20.425 |
36.585 |
45.310 |
55.495 |
|
Standard deviation |
0.120 |
1.534 |
0.085 |
0.007 |
|
1 mg mL−1 ι-carrageean in 49 % sucrose solution |
||||
|
Mean ice crystal diameter x1,0 [μm] |
16.725 |
24.355 |
27.080 |
31.095 |
|
Standard deviation |
0.332 |
1.959 |
2.135 |
1.747 |
|
1 mg mL−1 κ-carrageean in 49 % sucrose solution |
||||
|
Mean ice crystal diameter x1,0 [μm] |
8.040 |
8.975 |
9.355 |
10.795 |
|
Standard deviation |
0.283 |
0.502 |
0.007 |
0.007 |
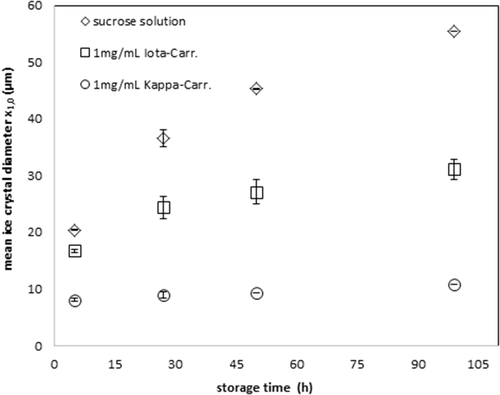
The mean ice crystal diameter was determined to be 55.495 µm in the sucrose solution while this value decreases to 31.095 µm and 10.795 µm in the presence of ι- and κ-carrageenans, respectively, at a storage time of 99 h. These relative results of the IRI activities of carrageenans allow revealing the influence of ι-carrageenan on IRI more clearly. In conclusion, the IRI effect of carrageenan solutions is higher for κ-carrageenan than for ι-carrageenan.
In the literature, the morphological change of ice crystals in the presence of κ-carrageenan has been investigated to explain the increased IRI activity 13. The photos of ice crystals grown in sucrose solution, in the presence of κ- and ι-carrageenans after a storage time of 50 h are presented in Fig. 3.
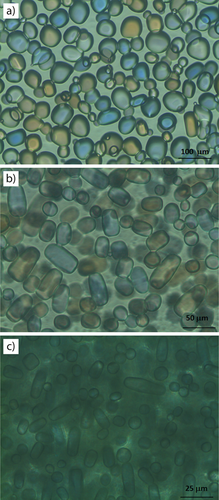
The round shape of the ice crystals was transformed into a more angular and elongated shape in the presence of κ-carrageenan. The same tendency is observed in the presence of ι-carrageenan. One of the shape parameters, namely circularity, can reflect the differences in morphology. The circularity value was determined by using the software ImagePro Insight 9.1 as 0.82 ± 0.009, 0.79 ± 0.035, and 0.76 ± 0.005 in sucrose solution and in the presence of κ-carrageenan and ι-carrageenan, respectively. A perfect circle has a circularity value of 1.0 while this value approaches to zero with increasing elongated shape. Therefore, as expected, the results demonstrate that round-shaped crystals have a high circularity, while more elongated and angular crystals have a low circularity. The decrease in circularity value confirms the morphology results. These changes in the shape of the crystals reveal that there is a connection between shape modification and IRI activity of carrageenans, which confirms the results found by Leiter et al. 14.
3.2 Thermal Hysteresis Activity of Carrageenans and Morphology of a Single Ice Crystal
The growth habit of ice crystals in sucrose solution is similar to that of ice crystals grown in pure water. The typical shape of an ice crystal grown in 40 % sucrose solution is illustrated in Fig. 4-1. As can be seen, the ice crystal is formed in a round shape as observed during recrystallization measurements (Fig. 4-1a). The top view of the ice crystal seen in Fig. 4-1 represents the growth of an ice crystal along the a-axis. When the temperature is reduced further, the crystal approaches a snow crystal shape. The side view of an ice crystal is illustrated in Fig. 4-2. When both views of the ice crystals are examined, it is clearly seen that the crystals grow along the a-axis. Growth in c-axis direction is not observed.
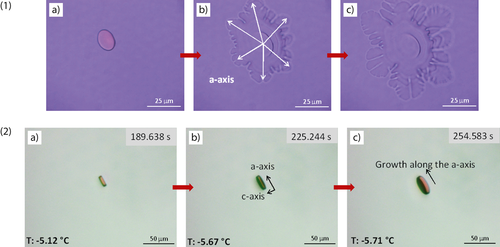
The TH measurements were performed in the presence of κ- and ι-carrageenans, which are expected to possess the TH activities and to affect the morphology of the ice crystals. Fig. 5 displays ice crystal growth in the presence of 1 mg mL−1 ı-carrageenan in 40 % sucrose solution. Ice crystal growth along the a-axis and angular and elongated shape are observed in the presence of ι-carrageenan. The ice crystal shape was modified but bursting is not observed in the presence of ι-carrageenan.

Photos of ice crystals growing during TH measurement in the presence of 10 mg.mL−1 κ-carrageenan in 40 % sucrose solution are shown in Fig. 6.

The round-shaped ice crystals obtained in the absence of κ-carrageenan were transformed into a more angular and elongated shape in the presence of κ-carrageenan. As a result, the same modification tendency is observed in the presence of both ι- and κ-carrageenan. The change in morphology of the growing ice crystals is observed in the presence of κ-carrageenan as emphasized by Gaukel et al. 13. Even if the growth habit modification happened in the presence of κ-carrageenan, there is not detected bursting in the presence of κ-carrageenan. The photos of the ice crystals grown in the presence of κ-carrageenan were taken from different viewpoints to further analyze the results in terms of growth habit modification. The morphology change of ice crystals grown in the presence of 10 mg mL−1 of κ-carrageenan is presented in Fig. 7.

As in the absence of κ-carrageenan (Fig. 4), the ice crystals grow in the a-axis direction whereas there is no growth along the c-axis (see Fig. 7). The crystal in the bottom left-hand corner can be observed from the first picture to the last. Burst growth is not detected. The crystals grow slowly along the a-axis.
Besides a suggestion that there is a connection between strong shape modification and TH activity, Peltier et al. revealed the effect of analogue concentration on the shape modification and, hence, on TH. They carried out TH measurements of analogues of AFGP8 synthesized by the authors. The same shape modification tendency seen in Fig. 6 has been detected at a low concentration of analogue 13 (10 mg mL−1) without TH. When the concentration of analogue 13 was increased, it was noticed that the shape modification is related to the concentration. The shape of the ice crystals was found to be a thin hexagonal plate, hexagonal rod, and truncated hexagonal bipyramid in the presence of analogue 13 at a concentration of 10, 20, and 40 mg mL−1, respectively, without TH.
At a high concentration of 80 mg mL−1, the ice crystal was in the hexagonal bipyramid-like shape, with a TH of 0.15 °C 22. Therefore, the growth of ice crystals in the presence of various concentrations of κ- and ι-carrageenans was examined. The same morphology leads to the conclusion that there is no influence of the concentration of carrageenans on the morphology of ice crystals. The growth of ice crystals in different solutions (Fig. 4-6) indicated that the presence of κ- and ι-carrageenans has an effect on the shape of the ice crystals but they do not have TH activity. As revealed in Sect. 3.1, κ- and ι-carrageenans significantly reduce recrystallization in a sucrose solution. These results demonstrate that κ- and ι-carrageenans do not possess TH activity despite of having a high IRI activity. These two properties point out that κ- and ι-carrageenans have great potential as novel cryoprotectants.
As pointed out in the literature, there is no correlation between IRI activity and TH activity 23, 24. Chow-Shi-Yee et al. reported that some wheat proteins provide only IRI activity with no TH activity 3. Gaukel et al. also examined the IRI activity of mixtures of AFPs and hydrocolloids. They showed that the IRI effect of protein solutions with κ-carrageenan increased 13. Based on this study, a possible influence of the presence of κ-carrageenan on the TH activity of AFGP was investigated. TH measurements were performed in 40 % sucrose solution in the presence of 1 mg mL−1 of AFGP and 1 mg mL−1 of AFGP together with 5 mg mL−1 of κ-carrageenan. The TH activity was measured as 0.82 °C with 0.01 °C standard deviation which was the same result obtained in the sole presence of 1 mg mL−1 of AFGP.
Although, the presence of κ-carrageenan with AFGP in the same solution resulted in a totally different IRI activity 13, the mixture of them had the same TH activity. This is also evidence of the fact that a TH activity does not relate to the strength of the IRI activity. The same crystal shape obtained in the presence of AFGP in 40 % sucrose solution is observed in the presence of κ-carrageenan and AFGP in 40 % sucrose solution. The shape of a round ice crystal transformed into the shape of hexagonal bipyramidal, and then burst as illustrated in Fig. 8.

4 Conclusion
The IRI and TH activities of κ- and ι-carrageenans were measured in sucrose solution. The IRI results reveal that ι-carrageenan significantly reduces recrystallization in a sucrose solution but is not as active as κ-carrageenan. The crystal shape changed from round to angular and elongated shape in the presence of κ-carrageenan. The same modification was observed in the presence of ι-carrageenan. These changes reveal that κ- and ι-carrageenans interact with specific ice planes. The TH measurements, however, demonstrate that κ- and ι-carrageenans do not possess TH activity. In addition, the presence of κ-carrageenan does not affect the TH activity of AFGP and the morphology of ice crystals grown in the presence of AFGP.
TH activity, which is an undesirable property for a good cryoprotectant, is still determined for AFGP even in the presence of κ-carrageenan. These results indicate that AFGP cannot be used as cryoprotectant even in the presence of κ-carrageenan while κ- and ι-carrageenans, which exhibit IRI activity and no TH activity, have desirable properties for a good cryoprotectant. In conclusion, κ- and ι-carrageenans can be employed as novel cryoprotectants in cryopreservation applications.
Acknowledgements
Financial support by the Scientific and Technological Research Council of Turkey (2219-International Postdoctoral Research Fellowship Program 2018/2, 1059B191800970) is gratefully acknowledged.
The authors have declared no conflict of interest.
Symbols used
-
- h [h]
-
storage time
-
- Tf [°C]
-
non-equilibrium freezing temperature
-
- Tm [°C]
-
melting temperature
-
- x1,0 [μm]
-
mean ice crystal diameter
Abbreviations
-
- AFGP
-
antifreeze glycoprotein
-
- AFP
-
antifreeze protein
-
- DMSO
-
dimethyl sulfoxide
-
- IBP
-
ice-binding protein
-
- IRI
-
ice recrystallization inhibition
-
- ISP
-
ice structuring protein
-
- ID
-
inner diameter
-
- OD
-
outer diameter
-
- TH
-
thermal hysteresis




