Association of cardiorenal biomarkers with mortality in metabolic syndrome patients: A prospective cohort study from NHANES
Abstract
Objectives
Approximately 20%–25% of the global adult population is affected by metabolic syndrome (MetS), highlighting its status as a major public health concern. This study aims to investigate the predictive value of cardiorenal biomarkers on mortality among patients with MetS, thus optimizing treatment strategies.
Methods
Utilizing data from the National Health and Nutrition Examination Survey (NHANES) cycles between 1999 and 2004, we conducted a prospective cohort study involving 2369 participants diagnosed with MetS. We evaluated the association of cardiac and renal biomarkers with all-cause and cardiovascular disease (CVD) mortality, employing weighted Cox proportional hazards models. Furthermore, machine learning models were used to predict mortality outcomes based on these biomarkers.
Results
Among 2369 participants in the study cohort, over a median follow-up period of 17.1 years, 774 (32.67%) participants died, including 260 (10.98%) from CVD. The highest quartiles of cardiac biomarkers (N-terminal pro-B-type natriuretic peptide [NT-proBNP]) and renal biomarkers (beta-2 microglobulin, [β2M]) were significantly associated with increased risks of all-cause mortality (hazard ratios [HRs] ranging from 1.94 to 2.06) and CVD mortality (HRs up to 2.86), after adjusting for confounders. Additionally, a U-shaped association was observed between high-sensitivity cardiac troponin T (Hs-cTnT), creatinine (Cr), and all-cause mortality in patients with MetS. Machine learning analyses identified Hs-cTnT, NT-proBNP, and β2M as important predictors of mortality, with the CatBoost model showing superior performance (area under the curve [AUC] = 0.904).
Conclusion
Cardiac and renal biomarkers are significant predictors of mortality in MetS patients, with Hs-cTnT, NT-proBNP, and β2M emerging as crucial indicators. Further research is needed to explore intervention strategies targeting these biomarkers to improve clinical outcomes.
Key points
-
Cardiac and renal biomarkers are significantly associated with metabolic syndrome (MetS) mortality.
-
High-sensitivity cardiac troponin T (Hs-cTnT) and creatinine show a U-shaped association with all-cause mortality risk among MetS patients.
-
Hs-cTnT, N-terminal pro-B-type natriuretic peptide, and beta-2 microglobulin are effective biomarkers for predicting death in MetS.
1 INTRODUCTION
Metabolic syndrome (MetS) represents a condition marked by the convergence of various cardiovascular and metabolic risk factors.1 This definition encompasses a range of risk factors, including elevated blood pressure, abnormal blood sugar, abnormal lipid metabolism, and central obesity.2 The collective impact of these factors increases the risk of cardiocerebrovascular diseases and diabetes. Hypertension stands out as the most critical component in MetS,3 closely intertwined with insulin resistance and abnormal lipid metabolism.4-6 In 2012, about one-third of the adult population in the United States (US) suffered from MetS.7 Globally, the prevalence of MetS stands at 20%–25%, signifying an escalating concern for public health.8 One study was conducted from 1999 to 2014, the all-cause mortality and cardiocerebrovascular mortality in MetS patients were 14.5% and 2.9%, respectively.9 MetS is significantly associated with increased mortality, especially cardiovascular disease (CVD) mortality.10
Cardiac biomarkers, such as high-sensitivity cardiac troponin T and I (Hs-cTnT and Hs-cTnI) and N-terminal pro-B-type natriuretic peptide (NT-proBNP), play a crucial role in evaluating heart function and assessing the risk of heart diseases.11-14 In patients with hypertension, sustained high blood pressure load can lead to changes in cardiac structure and function, increasing the risk of myocardial cell damage.15 Consequently, elevated levels of Hs-cTnT and Hs-cTnI might accelerate the progression of MetS and thus increase the mortality risk, especially in cases of MetS combined with hypertension. However, the robustness of this association, particularly in predicting the death risk in MetS, is not well-established due to insufficient evidence. Research by Pokharel indicates that the mortality risk in MetS associated with Hs-cTnT levels correlates with its components, highlighting uncertainty in the predictive outcomes of the biomarker levels for varying numbers of MetS components.16 In emergency medical situations, Hs-cTnT level is commonly employed as a predictor of CVD mortality risk. Studies on the long-term effectiveness of this biomarker in metabolic disorders and cardiovascular risk assessment are still limited.
Likewise, renal biomarkers, such as creatinine (Cr), cystatin C (CysC), and beta-2 microglobulin (β2M), serve as pivotal instruments in evaluating kidney function and monitoring the risk of chronic kidney disease (CKD).17-19 Increasing evidence suggests that renal dysfunction is a significant factor in the increased death risk of patients with MetS,20, 21 especially considering the high risk of CVD. However, the accuracy and consistency of these biomarkers in predicting the mortality risk in MetS patients remain uncertain. Therefore, precise identification of cardiac and renal biomarker levels in MetS patients could help in formulating intensified treatment and prevention strategies for these patients. Additionally, this could enable researchers to identify individuals at high risk.
In this context, though the diagnostic criteria for MetS have been clearly established, the identification of effective biomarkers for predicting its mortality risk remains a challenge. The importance of cardiac and renal biomarkers in mortality risk prediction in MetS patients has not been accessed. This study aims to investigate the role of cardiac and renal biomarkers in predicting the mortality risk among patients with MetS. We hypothesize that a combination of these biomarkers may offer more insights into long-term health outcomes in patients with MetS, promising not only to optimize treatment options for patients and provide a more comprehensive biomarker framework for risk assessment and management of MetS but also to help reduce future risk of CVD and diabetes.
2 METHOD
2.1 Study population
The National Health and Nutrition Examination Survey (NHANES) is a multi-cycle, cross-sectional survey that integrates population-specific health questionnaires and physical examinations to assess the health and nutritional status of adults and children in the United States. The survey uses a complex, stratified, multistage probability sampling method, ensuring that the selected samples can effectively represent the entire US population.22 This study used data from three survey cycles from 1999 to 2004, involving the recruitment of 31,126 participants. Individuals under 18 years of age (n = 14,065), those who reported pregnancy (n = 715), those missing fasting blood sample data (n = 9890), those with fewer than two metabolic abnormality indicators (n = 3662), those missing cardiac and renal biomarker data (n = 423), and those missing death data (n = 2) were excluded, resulting in a final sample of 2369 participants (Supporting Information: Figure S1). The NHANES program has been approved by the Institutional Review Board of the National Center for Health Statistics (NCHS) and participants provided signed informed consent forms.
2.2 MetS
According to the criteria set forth by the American College of Endocrinology (ACE) and the American Association of Clinical Endocrinologists (AACE), MetS is considered present when three or more of the following five conditions exist2: (1) increased waist circumference: ≥88 cm in women and ≥102 cm in men; (2) elevated triglycerides: ≥150 mg/dL; (3) low high-density lipoprotein cholesterol (HDL-C): <40 mg/dL in men and <50 mg/dL in women; (4) elevated blood pressure: systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mmHg, or self-reported use of antihypertensive medication; and (5) elevated fasting plasma glucose (FPG): FPG ≥ 100 mg/dL or use of glucose-lowering drugs.
Clinical data for MetS indicators were obtained at the mobile examination centers (MEC). Blood samples were collected from participants who had fasted for ≥8 h to determine FPG, HDL-C, and serum triglyceride concentrations. Waist circumference and blood pressure were measured using standard methods. After the participant had rested in a quiet sitting position for 5 min, blood pressure was measured on the right arm using a mercury sphygmomanometer, with three consecutive readings taken. If needed, take the fourth reading.
2.3 Cardiorenal biomarkers
During the NHANES 1999–2004 survey cycles, measurements of Hs-cTnT, Hs-cTnI, NT-proBNP, β2M, CysC, and Cr were conducted on stored residual serum samples. The limit of detection (LOD) for Hs-cTnT was 3 ng/L, with a total imprecision expressed as a coefficient of variation between 2.0% and 3.1%. The LOD for Hs-cTnI was 1.6 ng/L, with coefficients of variation of 3.8% (12–28 ng/L), 2.7% (120–280 ng/L), and 2.6% (9000–21,000 ng/L). NT-proBNP (Roche Diagnostics) had detection limits of 5 and 35,000 pg/mL, with a coefficient of variation of 2.7%–3.1%. Serum β2M and CysC were measured on the automated multi-channel analyzer Siemens Dimension Vista 1500 (Siemens Healthcare Diagnostics). The detection limits for β2M were 0.72 and 23.0 mg/L, with coefficients of variation of 3.42%–3.88%. Cystatin C had detection limits of 0.23 and 8.00 mg/L, with coefficients of variation of 3.54%–4.36%. Serum creatinine was determined using the Jaffé rate reaction.
For the biomarkers NT-proBNP, β2M, and CysC, when the analysis results were below the lower LOD, they were imputed using the estimated value of the limit of detection divided by the square root of 2 (). For results above the upper LOD, impute with an estimated value equal to the upper LOD value. Overall, NT-proBNP measurements for 87 individuals were imputed as , β2M measurements for one individual each were imputed as and upper LOD, respectively, and additionally, CysC measurements for 3 individuals were imputed as .
2.4 Covariates
Covariates were acquired through standardized questionnaires during interviews, including age, race, sex, marital status, education, poverty income ratio (PIR), smoking status, and drinking status. Marital status was categorized into married or living with partner, widowed or divorced or separated, and never married. Education was classified as less than high school (low), high school or equivalent (medium), and college graduate or above (high). PIR was divided into <1.0 and ≥1.0. Smoking status was categorized into never smoked, former smoker, and current smoker. Drinking status was divided into nondrinker, light drinker, moderate drinker, and heavy drinker. Height and weight were measured at the MEC, with body mass index (BMI) calculated as weight (kg) divided by height (m) squared. The determination of CVD history is made by answering the question, “Ever told had congestive heart failure/coronary heart disease/angina or angina pectoris/heart attack/stroke?” CKD is defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m². The eGFR is calculated using the CKD-EPI 2021 equation based on serum Cr and CysC.23 Diabetes is defined as plasma HbA1c ≥ 6.5% or FPG ≥ 126 mg/dL, self-reported previous diagnosis of diabetes, or use of insulin or oral hypoglycemic medication.24 The diagnosis of cancer is based on the question, “Have you ever been told you had cancer or any type of malignant tumor?” Missing covariates were imputed using multiple imputation methods.
2.5 Ascertainment of mortality
All individuals included in the study were followed up. Mortality from all causes and CVD was determined by matching with the national death index (NDI) up to December 31, 2019. The primary causes of death were identified based on ICD-10 codes. CVD mortality was defined as deaths caused by heart diseases (I00–I09, I11, I13, I20–I51) and cerebrovascular diseases (I60–I69).25
2.6 Statistical analysis
Baseline characteristics of participants were compared by outcome event occurrence. Continuous variables with a normal distribution are presented as means with standard deviations (SD), whereas those with a nonnormal distribution are presented as medians with interquartile ranges (IQR). Categorical variables are expressed in frequencies and percentages. Between-group differences for continuous variables were assessed using the Student's t-test or Wilcoxon rank–sum test, whereas the χ2 test or Fisher's exact test for categorical variables.
Univariate and multivariate weighted Cox proportional hazards models were employed to assess the associations of Hs-cTnT (continuous and quartiles), Hs-cTnI (continuous and quartiles), NT-proBNP (continuous and quartiles), Cr (continuous and quartiles), β2M (continuous and quartiles), and CysC (continuous and quartiles) with mortality from MetS. Considering the skewed distribution of variables, log-transformed biomarkers were used for regression analysis. Three models were formulated: Model 1 without adjustment; Model 2 adjusted for age, sex, and race; Model 3 adjusted for age, sex, race, marital status, PIR, education, drinking status, smoking status, BMI, CVD, CKD, diabetes, and cancer. Hazard ratio (HR) and 95% confidence interval (CI) were used to assess the risk in the models. Additionally, restricted cubic spline (RCS) curves based on multivariate weighted Cox regression were plotted to visualize the linear or nonlinear relationships between biomarkers and all-cause mortality, and CVD mortality. We also performed subgroup analysis by potential confounders.
Five machine learning models, random forest (RF), support vector machine (SVM), categorical boosting (CatBoost), eXtreme gradient boosting (XGBoost), and light gradient boosting machine (LightGBM), were employed to predict mortality from MetS using cardiac and renal biomarkers. The data were split into a training set (70%, 1658 individuals) and a test set (30%, 711 individuals). Models performance metrics included area under the curve (AUC), accuracy, precision, recall, and F1 score for both the training and test sets. Based on the best machine learning model from the training and test sets, the relative importance of cardiac and renal biomarkers was determined using Shapley Additive exPlanation (SHAP) values. A beewarm plot is a more complex and informative representation based on SHAP values, which not only indicates the relative importance of features but also reveals their actual relationship with the predicted outcome. The top three biomarkers were selected for incorporating into the final predictive model, combined with confounding variables, to construct the optimal model for predicting the mortality risk from MetS.
All analyses were performed using R software, with a two-tailed test, and a p-value < 0.05 was considered statistically significant.
3 RESULTS
3.1 Baseline characteristics
In 2369 NHANES study population (weighted population 75,042,131), there were 1157 males and 1229 non-Hispanic Whites (Table 1). Among the MetS patients who experienced outcome events, 602 had elevated blood pressure and were more likely to have underlying conditions. Median and IQR levels of serum Cr by outcome event occurrence were 70.72 [61.90–88.40] µmol/L, 79.56 [61.90–97.24] µmol/L, respectively, with no significant differences (p > 0.05). Significant differences were observed in the median levels of Hs-cTnT, Hs-cTnI, NT-proBNP, CysC, and β2M across different groups (p < 0.001). Furthermore, there were no significant differences in Cr across different survey periods (Supporting Information: Table S1). At the same time, we compare the baseline characteristics of the included and excluded populations (Supporting Information: Table S2). The two groups showed no significant differences in terms of gender, educational level, and drink status (p > 0.05), indicating that the two groups are comparable in these aspects.
| Characteristics | Total (n = 2369) | Without outcome events (n = 1595) | With outcome events (n = 774) | p-Value |
|---|---|---|---|---|
| Age (years) | 51.86 (19.69) | 43.52 (16.7) | 69.04 (13.1) | <0.001 |
| Gender | ||||
| Male | 1157 (48.8) | 746 (46.8) | 411 (53.1) | <0.05 |
| Female | 1212 (51.2) | 849 (53.2) | 363 (46.9) | |
| Race/ethnicity | <0.001 | |||
| Mexican American | 565 (23.8) | 406 (25.5) | 159 (20.5) | |
| Other Hispanic | 90 (3.7) | 59 (3.7) | 31 (4.0) | |
| Non-Hispanic White | 1229 (51.8) | 780 (48.9) | 449 (58.0) | |
| Non-Hispanic Black | 408 (17.2) | 292 (18.3) | 116 (15.0) | |
| Other Race | 77 (3.2) | 58 (3.6) | 19 (2.5) | |
| Education | <0.001 | |||
| ≤11th grade | 808 (34.1) | 492 (30.8) | 316 (40.8) | |
| High school graduate or equivalent | 607 (25.6) | 419 (26.3) | 188 (24.3) | |
| More than high school | 954 (40.3) | 684 (42.9) | 270 (34.9) | |
| Marital status | <0.001 | |||
| Married/Living with partner | 1409 (59.5) | 967 (60.6) | 442 (67.1) | |
| Widowed/Divorced/Separated | 526 (22.2) | 245 (15.4) | 281 (36.3) | |
| Never married | 434 (18.3) | 383 (24.0) | 51 (6.6) | |
| PIR | 0.18 | |||
| ≤1 | 422 (17.8) | 272 (17.1) | 150 (19.4) | |
| >1 | 1947 (82.2) | 1323 (82.9) | 624 (80.6) | |
| Drink status | <0.001 | |||
| Never | 494 (20.8) | 294 (18.4) | 200 (25.8) | |
| Light | 685 (28.9) | 462 (29.0) | 223 (28.8) | |
| Moderate | 473 (20.0) | 353 (22.1) | 120 (15.5) | |
| Heavy | 717 (30.3) | 486 (30.5) | 231 (29.8) | |
| Smoke status | <0.001 | |||
| Never | 1180 (49.8) | 855 (53.6) | 325 (42.0) | |
| Former | 700 (29.5) | 376 (23.6) | 324 (41.9) | |
| Current | 489 (20.7) | 364 (22.8) | 125 (16.1) | |
| BMI (kg/m2) | <0.001 | |||
| <18.5 | 27 (1.1) | 20 (1.3) | 7 (0.9) | |
| 18.5–24.9 | 593 (25.0) | 439 (27.5) | 154 (19.9) | |
| 25–30 | 802 (33.9) | 517 (32.4) | 285 (36.8) | |
| ≥30 | 947 (40.0) | 619 (38.8) | 328 (42.4) | |
| MetS component | <0.001 | |||
| Increased waist circumference | 1466 (61.9) | 906 (56.8) | 560 (72.4) | |
| Elevated triglycerides | 1047 (44.2) | 632 (39.6) | 415 (53.6) | |
| Low HDL-C | 967 (40.8) | 645 (40.4) | 322 (41.6) | |
| Elevated blood pressure | 1310 (55.3) | 708 (44.4) | 602 (77.8) | |
| Elevated FPG | 1240 (52.3) | 689 (43.2) | 551 (71.2) | |
| CVD | 308 (13.0) | 96 (6.0) | 212 (27.4) | <0.001 |
| CKD | 182 (7.7) | 16 (1.0) | 166 (21.4) | <0.001 |
| Diabetes | 416 (17.6) | 178 (11.2) | 238 (30.7) | <0.001 |
| Cancer | 213 (9.0) | 79 (5.0) | 134 (17.3) | <0.001 |
| Hs-cTnT (ng/L) | 6.28 [4.34–10.3] | 5.14 [3.81–7.28] | 11.21 [7.22–17.88] | <0.001 |
| Hs-cTnI (ng/L) | 3.23 [1.7–6.08] | 2.54 [1.38–4.46] | 5.46 [3.11–10.22] | <0.001 |
| NT-proBNP (pg/mL) | 52.49 [22.3–122] | 37.03 [16.87–71.10] | 133.35 [58.43–313.30] | <0.001 |
| β2M (mg/L) | 1.99 [1.68–2.43] | 1.83 [1.60–2.12] | 2.48 [2.06–3.19] | <0.001 |
| CyC (mg/L) | 0.78 [0.68–0.91] | 0.73 [0.65–0.83] | 0.92 [0.80–1.13] | <0.001 |
| Cr (μmol/L) | 70.72 [61.9–88.4] | 70.72 [61.9–88.4] | 79.56 [61.9–97.24] | 0.46 |
| Follow-up time (years) | 17.08 [14.2–18.4] | 17.83 [16.93–18.75] | 9.67 [5.58–14.00] | <0.001 |
- Note: Data are presented as mean ± SD, n (%), or median and IQR.
- Abbreviations: β2M, beta-2 microglobulin; BMI, body mass index; CKD, chronic kidney disease; Cr, creatinine; CVD, cardiovascular disease; CysC, cystatin C; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; Hs-cTnT, high-sensitivity cardiac troponin T; Hs-cTnI, high-sensitivity cardiac troponin L; MetS, metabolic syndrome; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
3.2 Associations between cardiorenal biomarkers and mortality risk
During the follow-up, a total of 774 (32.67%) participants died from various causes, including 260 (10.98%) CVD-related deaths. Table 2 shows the relationship between different cardiorenal biomarkers and all-cause mortality. After multivariable adjustment (Model 3), compared to the control group (Q1), the highest quartile of NT-proBNP, and β2M were associated with an increased risk of all-cause mortality by 106% (HR 2.06, 95% CI 1.50–2.83), 94% (HR 1.94, 95% CI 1.24–3.05), respectively. In addition, a similar association was found between cardiorenal biomarkers and CVD mortality (Table 3). After adjusting for potential confounders (Model 3), the HRs for the highest quartile of Hs-cTnI, NT-proBNP, and β2M compared with the control group (Q1) were 2.31 (95% CI 1.25–4.28), 2.86 (95% CI 1.26–6.48), and 2.80 (95% CI 1.09–7.18), respectively.
| Biomarker | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| Log-Hs-cTnT | 19.29 (12.7–29.3)** | 3.35 (1.99–5.62)** | 1.91 (1.11–3.27)* |
| Q1 | Ref | Ref | Ref |
| Q2 | 1.39 (0.97–1.99) | 0.74 (0.50–1.09) | 0.74 (0.50–1.11) |
| Q3 | 3.88 (2.80–5.37)** | 0.92 (0.63–1.38) | 0.86 (0.57–1.31) |
| Q4 | 10.8 (7.70–15.11)** | 1.53 (0.99–2.36) | 1.19 (0.74–1.91) |
| ptrend | <0.01 | <0.01 | >0.05 |
| Log-Hs-cTnI | 3.07 (2.34–4.02)** | 1.59 (1.31–1.93)** | 1.33 (1.08–1.64)** |
| Q1 | Ref | Ref | Ref |
| Q2 | 1.65 (1.08–2.53)* | 0.80 (0.53–1.21) | 0.77 (0.50–1.19) |
| Q3 | 2.94 (2.10–4.13)** | 0.86 (0.64–1.16) | 0.80 (0.59–1.08) |
| Q4 | 5.79 (4.43–7.55)** | 1.55 (1.17–2.06)** | 1.24 (0.92–1.65) |
| ptrend | <0.01 | <0.01 | <0.01 |
| Log-NT-proBNP | 5.35 (4.43–6.45)** | 2.21 (1.86–2.62)** | 1.89 (1.55–2.29)** |
| Q1 | Ref | Ref | Ref |
| Q2 | 1.50 (1.06–2.13)* | 1.02 (0.67–1.56) | 1.09 (0.71–1.65) |
| Q3 | 2.76 (2.00–3.80)** | 1.29 (0.93–1.78) | 1.32 (0.97–1.78) |
| Q4 | 9.28 (6.86–12.55)** | 2.28 (1.64–3.17)** | 2.06 (1.50–2.83)** |
| ptrend | <0.01 | <0.01 | <0.001 |
| Log-Cr | 17.9 (6.16–51.9)** | 2.70 (0.76–9.56) | 0.68 (0.14–3.33) |
| Q1 | Ref | Ref | Ref |
| Q2 | 1.28 (0.92–1.76) | 0.81 (0.60–1.11) | 0.86 (0.62–1.18) |
| Q3 | 1.32 (0.93–1.87) | 0.71 (0.50–1.01) | 0.71 (0.49–1.04) |
| Q4 | 3.38 (2.47–4.63)** | 1.18 (0.86–1.62) | 0.89 (0.62–1.28) |
| ptrend | <0.01 | >0.05 | >0.05 |
| Log-CysC | 261.2 (78.98–863.6)** | 21.34 (9.36–48.67)** | 7.01 (2.14–22.99)** |
| Q1 | Ref | Ref | Ref |
| Q2 | 1.62 (1.00–2.61)* | 1.06 (0.66–1.73) | 1.14 (0.68–1.91) |
| Q3 | 3.50 (2.15–5.71)** | 1.29 (0.77–2.16) | 1.29 (0.76–2.21) |
| Q4 | 10.21 (6.77–15.40)** | 1.69 (1.09–2.63)* | 1.35 (0.83–2.17) |
| ptrend | <0.01 | <0.01 | >0.05 |
| Log-β2M | 109 (34.8–341.6)** | 29.76 (16.34–54.20)** | 20.75 (9.47–45.48)** |
| Q1 | Ref | Ref | Ref |
| Q2 | 2.03 (1.31–3.15)** | 1.12 (0.77–1.63) | 1.15 (0.78–1.70) |
| Q3 | 3.94 (2.36–6.57)** | 1.31 (0.79–2.16) | 1.29 (0.77–2.16) |
| Q4 | 12.62 (8.41–18.96)** | 2.32 (1.52–3.53)** | 1.94 (1.24–3.05)** |
| ptrend | <0.01 | <0.01 | <0.01 |
- Note: Model 1: Unadjusted model. Model 2: Adjusted for age, race, and gender. Model 3: Model 2+ education, marital status, PIR, smoke status, drink status, BMI, CVD, CKD, diabetes, and cancer.
- Abbreviations: β2M, beta-2 microglobulin; BMI, body mass index; CKD, chronic kidney disease; CI, confidence interval; Cr, creatinine; CysC, cystatin C; CVD, cardiovascular disease; HR, hazard ratio; Hs-cTnT, high-sensitivity cardiac troponin T; Hs-cTnI, high-sensitivity cardiac troponin L; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
- ** p < 0.01
- * p < 0.05.
| Biomarker | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| Log-Hs-cTnT | 29.71 (16.81–52.53)** | 6.09 (3.29–11.30)** | 2.79 (1.18–6.63)* |
| Q1 | Ref | Ref | Ref |
| Q2 | 2.60 (1.02–6.60)* | 1.37 (0.52–3.61) | 1.36 (0.49–3.79) |
| Q3 | 8.28 (3.34–20.50)** | 1.92 (0.72–5.12) | 1.67 (0.59–4.69) |
| Q4 | 25.38 (9.92–64.92)** | 3.47 (1.28–9.40)* | 2.33 (0.79–6.85) |
| ptrend | <0.01 | <0.01 | <0.05 |
| Log-Hs-cTnI | 4.21 (2.83–6.26)** | 2.59 (1.90–3.53)** | 2.07 (1.45–2.95)** |
| Q1 | Ref | Ref | Ref |
| Q2 | 2.81 (1.29–6.12)** | 1.29 (0.60–2.78) | 1.29 (0.58–2.85) |
| Q3 | 4.47 (2.19–9.14)** | 1.20 (0.61–2.36) | 1.07 (0.54–2.12) |
| Q4 | 13.67 (7.41–25.22)** | 3.36 (1.79–6.32)** | 2.31 (1.25–4.28)** |
| ptrend | <0.01 | <0.01 | <0.01 |
| Log-NT-proBNP | 7.43 (5.66–9.75)** | 3.17 (2.40–4.19)** | 2.34 (1.61–3.42)** |
| Q1 | Ref | Ref | Ref |
| Q2 | 1.88 (0.73–4.84) | 1.27 (0.50–3.21) | 1.31 (0.52–3.28) |
| Q3 | 3.54 (1.46–8.56)** | 1.61 (0.67–3.85) | 1.57 (0.66–3.78) |
| Q4 | 15.45 (6.62–36.03)** | 3.65 (1.61–8.25)** | 2.86 (1.26–6.48)* |
| ptrend | <0.01 | <0.01 | <0.01 |
| Log-Cr | 33.48 (7.90–141.9)** | 7.81 (1.79–34.02)** | 1.17 (0.26–5.15) |
| Q1 | Ref | Ref | Ref |
| Q2 | 1.97 (1.17–3.34)* | 1.20 (0.68–2.11) | 1.37 (0.80–2.36) |
| Q3 | 1.71 (0.96–3.04) | 0.86 (0.49–1.49) | 0.86 (0.50–1.50) |
| Q4 | 4.89 (2.89–8.25)** | 1.53 (0.86–2.73) | 0.96 (0.52–1.78) |
| ptrend | <0.01 | >0.05 | >0.05 |
| Log-CysC | 347.6 (80.91–1493)** | 31.65 (7.88–127.12)** | 5.04 (1.02–24.88)* |
| Q1 | Ref | Ref | Ref |
| Q2 | 2.41 (1.14–5.09)** | 1.50 (0.68–3.29) | 1.78 (0.81–3.88) |
| Q3 | 6.41 (2.70–15.23)** | 2.09 (0.83–5.27) | 2.36 (0.97–5.73) |
| Q4 | 17.46 (7.88–38.67)** | 2.32 (0.96–5.63) | 1.77 (0.80–3.93) |
| ptrend | <0.01 | <0.05 | >0.05 |
| Log-β2M | 128.8 (35.58–466.5)** | 37.77 (13.07–109.15)** | 15.00 (4.05–55.49)** |
| Q1 | Ref | Ref | Ref |
| Q2 | 3.46 (1.50–8.02)** | 1.78 (0.76–4.20) | 1.86 (0.76–4.60) |
| Q3 | 5.45 (2.44–12.18)** | 1.62 (0.67–3.88) | 1.65 (0.67–4.09) |
| Q4 | 22.84 (10.70–48.73)** | 3.50 (1.44–8.46)** | 2.80 (1.09–7.18)* |
| ptrend | <0.01 | <0.01 | <0.05 |
- Note: Model 1: unadjusted model. Model 2: adjusted for age, race, and gender. Model 3: model 2+ education, marital status, PIR, smoke status, drink status, BMI, CVD, CKD, diabetes, and cancer.
- Abbreviations: β2M, beta-2 microglobulin; BMI, body mass index; CKD, chronic kidney disease; CI, confidence interval; Cr, creatinine; CysC, cystatin C; CVD, cardiovascular disease; HR, hazard ratio; Hs-cTnT, high-sensitivity cardiac troponin T; Hs-cTnI, high-sensitivity cardiac troponin L; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PIR, poverty income ratio.
- ** p < 0.01
- * p < 0.05.
3.3 Dose–response relationship between cardiorenal biomarkers and mortality
We used RCS analysis to further explore the potential nonlinear associations between biomarkers and MetS mortality. The associations of biomarkers with all-cause mortality risk is visualized in Figure 1. Hs-cTnT, Hs-cTnI, Cr, and CysC showed significant nonlinear associations with all-cause mortality in MetS (p < 0.05 for nonlinearity), whereas NT-proBNP and β2M exhibited positive linear associations (p > 0.05 for nonlinearity). A nonlinear association was found only between Cr level and CVD mortality (p < 0.05 for nonlinearity, Supporting Information: Figure S2).
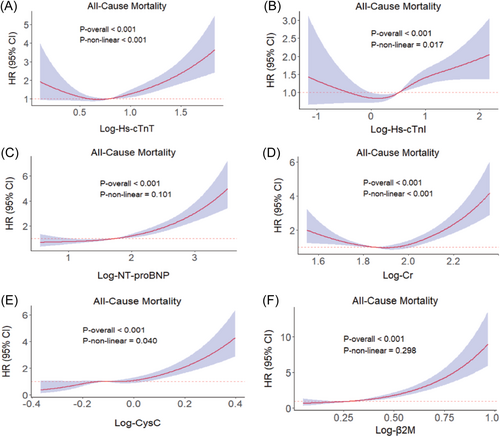
3.4 Subgroup analysis
In the subgroup analysis of all-cause mortality in patients with MetS and cardiorenal biomarkers (Supporting Information: Table S4), after multivariable adjustment, the risk of death in women aged ≥60 years increased with higher levels of Hs-cTnT, Hs-cTnI, NT-proBNP, and β2M (p < 0.05, HR > 1). Similar results were observed in the subgroup analysis of cardiovascular mortality (Supporting Information: Table S5). Furthermore, we applied Spearman's rank correlation to estimate the correlation between age and biomarkers (Supporting Information: Figure S3). All six biomarkers were positively correlated with age, with the correlation coefficient for NT-proBNP being 0.64 and for Cr being 0.18. Meanwhile, among patients with MetS without comorbidities, NT-proBNP was a risk factor for both all-cause and CVD mortality, whereas β2M and Hs-cTnI were risk factors for all-cause mortality and CVD mortality, respectively.
3.5 Performance comparison of machine learning models
To determine the optimal machine learning model for predicting mortality in MetS, the 2369 study subjects were randomly divided into a training set (70%, N = 1658) and a testing set (30%, N = 711). Through the comparison of the training set and the testing set, it is found that the baseline characteristics of the two groups of objects are comparable (Supporting Information: Table S3). Six biomarkers were included, Hs-cTnT, Hs-cTnI, NT-proBNP, Cr, CysC, and β2M, to construct five machine learning models, SVM, RF, XGBoost, LightGBM, and CatBoost. We utilized five evaluation metrics—AUC, accuracy, precision, recall, and F1 score—to assess the performance of these machine learning models in the training and testing cohorts. In the training set, the RF model exhibited the best predictive performance, with all evaluation metrics being 1. In the testing set, the CatBoost model demonstrated the highest AUC (0.862), accuracy (0.805), precision (0.832), recall (0.892), and F1 (0.861), followed by the SVM (0.858), RF (0.853), XGBoost (0.847), and LightGBM (0.828) models (Supporting Information: Table S6). Figure 2A shows the AUC value with 95% CI for five machine learning models, and Figure 2B,C shows the receiver operating characteristic (ROC) curves for RF and CatBoost, respectively.
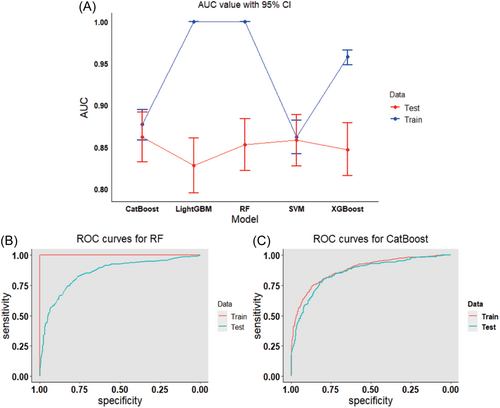
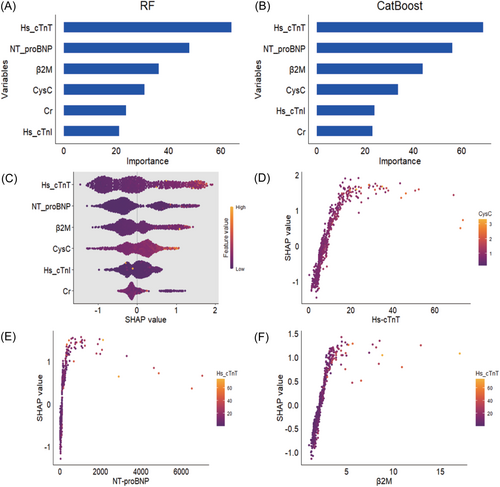
3.6 Global and local explanations of the machine learning models
To explain how machine learning models predict mortality in MetS, we aimed to elucidate the impact of each biomarker on the predictive models through variable importance and SHAP values. Ranking the variable importance based on the RF and CatBoost models, Hs-cTnT, NT-proBNP, and β2M emerged as the top three important variables in both models (Figure 3A–C). Figure 3D–F displays the dependency plots for the top three features determined by average absolute SHAP values, revealing similarities in the relationship between SHAP values and variable values across features.
3.7 CatBoost model for the combined assessment of biomarkers in predicting all-cause mortality
In the original model, the addition of Hs-cTnT, NT-proBNP, and β2M individually resulted in AUC of 0.901, 0.903, and 0.903, respectively (Supporting Information: Figure S4). The combinations of biomarkers, Hs-cTnT+NT-proBNP, Hs-cTnT+β2M, and NT-proBNP+β2M, showed minor improvements in model performance, with AUCs of 0.903, 0.904, and 0.904, respectively. Combining all three biomarkers, the CatBoost model's predictive performance was higher than that of the individual biomarkers and was significantly better than the original model, with an AUC of 0.904.
4 DISCUSSION
In this nationally representative prospective cohort study, significant associations were found between cardiac biomarkers (Hs-cTnT, Hs-cTnI, and NT-proBNP) and renal biomarkers (β2M, CysC, and Cr) with mortality in MetS. Additionally, a U-shaped association was observed between Hs-cTnT, Cr, and all-cause mortality in patients with MetS. Furthermore, by employing five advanced machine learning models—LightGBM, CatBoost, XGBoost, RF, and SVM—we successfully identified Hs-cTnT, NT-proBNP, and β2M as strong predictive indicators for mortality outcomes in patients with MetS. Currently, there is a lack of risk models for patients with MetS, especially predictive models that include biomarkers with strong prognostic value. These findings provide a new perspective on the relationship between cardiac and renal functions and the prognosis of patients with MetS.
Consistent with other studies, our data show that 55.3% of patients with MetS also have hypertension, indicating that elevated blood pressure is common among individuals with MetS.26, 27 Our findings suggest that cardiac biomarkers are of significant value in predicting mortality risk in patients with MetS, especially CVD mortality, which significantly increases within the highest quartiles of Hs-cTnI and NT-proBNP. Hs-cTnI is a marker of myocardial injury.28 In the context of MetS, Hs-cTnI can be used to improve the burden of diabetic complications,29 especially in individuals with CVD, identifying subgroups with a high mortality risk.30 Hs-cTnT exhibits a U-shaped association with all-cause mortality, where Hs-cTnT levels between 3.84 ng/L and 6.33 ng/L serve as a protective factor. Extremely low Hs-cTnT levels might indicate insufficient cardiac stress or a lack of necessary physiological responses, which could predispose individuals to adverse health outcomes.
Our findings are consistent with previous research, providing strong evidence for the prognostic value of NT-proBNP in populations with MetS.31-34 In Cox multivariate analysis, NT-proBNP was associated with both all-cause and CVD mortality. Furthermore, in subgroup analysis, high levels of NT-proBNP were identified as a risk factor for mortality in patients with MetS without a history of CVD. Therefore, elevated NT-proBNP may reflect underlying or overt CVD risk. Elevated natriuretic peptides indicate atrial or ventricular dilation due to pressure or volume overload, accelerating cardiac remodeling.35-37 Additionally, natriuretic peptides play significant roles in vascular function and remodeling. Other factors, such as endothelin, angiotensin II, and tumor necrosis factor-alpha, have been found to stimulate BNP secretion in vitro, increasing vascular reactivity and promoting CVD development.38 Moreover, there is a strong positive correlation between age and NT-proBNP, with older adults having significantly higher levels than younger individuals.39 The relative association of NT-proBNP with all-cause and CVD mortality remains consistent across age groups.
The renal biomarker β2M also demonstrated predictive value, with its highest quartile values being significantly associated with all-cause mortality and CVD mortality in MetS. Under normal conditions, the production rate of β2M is constant, and it is filtered by the glomeruli, then reabsorbed and degraded in the tubules, resulting in its minimal presence in blood and urine.40 β2M is metabolism-dependent and closely related to renal function, becoming an indicator for early diagnosis of renal function impairment.41 Previous studies have further underscored the importance of β2M in predicting adverse outcomes in MetS, especially as an indicator for the development of CKD. Over 9-year follow-up, patients with full MetS were 2.5 times more likely to develop CKD than those without it.42 HDL-C, one of the manifestations of dyslipidemia in MetS, plays a crucial role in anti-atherosclerosis and reducing the risk of CVD. Studies reveal a negative correlation between β2M levels and HDL-C, where high β2M indicates lower HDL-C and increased cardiovascular risk.43, 44 Measuring β2M holds significant value in identifying high-risk individuals within the MetS population, especially in the context of low HDL-C levels.
Machine learning algorithms, widely used for predicting new biomarkers and gaining new information about disease pathogenesis, analyze large amounts of health data to identify potential disease patterns.45, 46 By using machine learning models, we further strengthened the evidence of these biomarkers as independent predictors of mortality risk in patients with MetS. Feature importance analysis using RF and LightGBM models indicated that Hs-cTnT, NT-proBNP, and β2M are strong predictive indicators for mortality outcomes in patients with MetS. The addition of single and combined cardiac and renal biomarkers significantly improved model performance, with AUCs ranging from 0.901 to 0.904. This approach provides a more precise risk assessment tool, thereby helping to reduce disease risk and supporting personalized medicine.
Overall, the findings of this study emphasize the importance of cardiac and renal biomarkers in identifying cardiovascular event risk and assessing mortality risk in MetS, and demonstrate the potential of machine learning in risk assessment.
However, our study also has certain limitations. First, the sample size was relatively small, and the study population mainly came from specific geographical areas, which may restrict the generalizability of our findings. Future research should consider a more diverse population and longer follow-up times to enhance the relevance of these results. Additionally, this analysis was limited by the reliance on initial baseline measurements of biomarkers, which may diminish the accuracy of predictive models. Other health risk factors such as genetics, lifestyle, and individual medical history could also influence the interpretation of biomarkers. Finally, the classification of all-cause mortality and CVD mortality in NHANES was entirely based on ICD codes. Although this method has received validation from the Centers for Disease Control and Prevention (CDC) in the United States and is commonly used in many CDC reports or related published reports, we cannot rule out the possibility of errors in the cause of death determination.
AUTHOR CONTRIBUTIONS
Qianyi Gao wrote the first draft of the manuscript and revised the manuscript based on the authors' suggestions. Guideline panel members Shuanglong Jia, Huan Zhang, and Xingbo Mo critically reviewed the manuscript and provided additional information. Huan Zhang and Xingbo Mo were the chair of the panel and led the panel meeting. All authors approved the content.
ACKNOWLEDGMENTS
The study was supported by the National Natural Science Foundation of China (82173597 and 82073636), the Startup Fund from Soochow University (Q413900313 and Q413900412), and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The studies involving human participants were reviewed and approved by the Research Ethics Review Board of the NCHS. The patients/participants provided their written informed consent to participate in this study.
Open Research
DATA AVAILABILITY STATEMENT
Publicly available data sets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes.




