A Case of BRASH Syndrome Presenting With Refractory Hyperkalemia Requiring Intermittent Hemodialysis
Funding: The authors received no specific funding for this work.
Members of DOW are listed in the Acknowledgments section.
ABSTRACT
BRASH syndrome characterized by bradycardia, renal dysfunction, atrioventricular nodal blockade (AVNB), shock, and hyperkalemia presents diagnostic and management challenges due to its complex pathophysiology and varied clinical presentations. We describe a 90-year-old woman with a history of multiple comorbidities who was on beta blockers bisoprolol for heart failure, presented with shock, refractory hyperkalemia along with bradycardia that required intermittent hemodialysis. Initial management involved aggressive hyperkalemia medical therapy and fluid resuscitation, with subsequent consideration of renal replacement therapy hemodialysis following collaboration with a multidisciplinary team, including cardiology and nephrology specialists. Despite aggressive medical management for hyperkalemia, some cases of BRASH syndrome may remain challenging to treat, requiring intermittent hemodialysis highlighting the need for further research and understanding of this complex clinical entity to improve treatment outcomes.
Summary
- BRASH syndrome, involving bradycardia, renal dysfunction, AV nodal blockade, shock, and hyperkalemia, can present with severe, treatment-resistant hyperkalemia.
- We describe a 90-year-lady on beta blockers bisoprolol for heart failure, presented with shock, hyperkalemia, and bradycardia that required intermittent hemodialysis for effective management.
- Collaborative, multidisciplinary approaches are essential to optimize outcomes.
Abbreviations
-
- AV
-
- atrioventricular
-
- BRASH
-
- bradycardia, renal failure, AV nodal blockade, shock, and hyperkalemia
-
- ECG
-
- electrocardiogram
-
- GCS
-
- Glasgow Coma Scale
-
- ICU
-
- intensive care unit
1 Introduction
BRASH syndrome manifests as a clinical condition characterized by bradycardia, renal dysfunction, atrioventricular nodal blockade (AVNB), shock, and hyperkalemia. The clinical presentation of BRASH syndrome is usually dominated by manifestations of BRASH syndrome itself, rather than a precipitating event [1]. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, along with digitalis, increase the risk of hyperkalemia and renal dysfunction, while beta blockers such as atenolol and nadolol, which are renally excreted, demonstrate heightened accumulation during BRASH syndrome [1].
The pathology of this peculiar syndrome is a synergism between hyperkalemia and AVNB. Even mild dehydration can diminish renal perfusion and elevate the concentration of cardiovascular drugs metabolized by the kidneys, such as beta blockers and calcium channel blockers. This phenomenon can exacerbate bradycardia and renal failure, potentially leading to hyperkalemia. The presence of hyperkalemia and diminished renal clearance, resulting from delayed medication excretion, further compounds the issue, amplifying the effects of AV nodal blockers. Thus, a lethal vicious cycle could occur.
For early and effective management, recognition of multiple symptoms which may mimic various clinical entities is very crucial. Defining BRASH syndrome and exploring the pathophysiology may help optimize diagnosis and management, although most patients typically show improvement with basic supportive therapy. We present a case of refractory hyperkalemia landing with symptomatic bradycardia, leading to BRASH syndrome manifestation.
2 Case History/Presentation
A 90-year-old woman presented to the emergency department with complaints of shortness of breath (SOB), vomiting, and chest pain for one day. She has a medical history of Diabetes Mellitus-II (on metformin 500 mg twice daily), hypertension (on amlodipine 5 mg once daily and telmisartan 40 mg once daily) and heart failure with preserved ejection fraction (HFpEF) (on aspirin 75 mg once daily, bisoprolol 5 mg once daily, furosemide/spironolactone 20/50 mg once daily, and atorvastatin 10 mg once daily).
2.1 Investigation, Diagnosis, and Treatment
On presentation at the ER, her heart rate was 30 beats per minute, blood pressure was 70/40 mmHg, respiratory rate was 20 breaths per minute, and oxygen saturation was 95% on 2 L of oxygen. She was fully conscious with a Glasgow Coma Scale score (GCS) of 15. Routine laboratory parameters were sent. Her serum potassium was high, 7.5 mmol/L. Arterial blood gas analysis indicated metabolic acidosis with a pH of 7.2, bicarbonate of 13.9 mmol/L, and normal lactate level of 1.8 mmol/L. Her electrocardiogram (ECG) was remarkable (Figure 1), showing first-degree AV block with inverted T-wave and ST depression. The chest x-ray showed bilateral pleural effusions. Immediate intervention included intravenous atropine (0.6 mg) for bradycardia, regular insulin (8 units) /dextrose (50% 50 mL), salbutamol (5 mg) nebulization for hyperkalemia, and sodium bicarbonate (50 mEq) for acidosis. She was started on dopamine infusion at 5 μg per kg per min after initial fluid resuscitation.
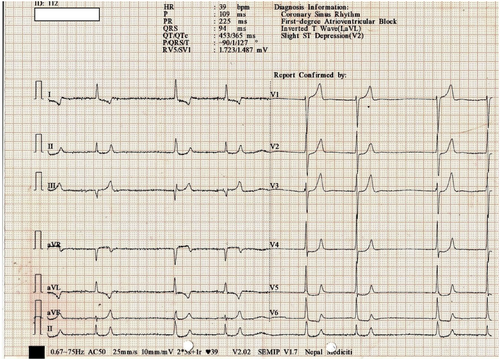
Initial ECG showing bradycardia with a rate of 39 bpm and first-degree AV block.
The patient was then immediately transferred to the intensive care unit (ICU) for further management. In the ICU, the diagnosis of BRASH syndrome was made as the patient fulfilled all five criteria: bradycardia, renal failure, AVNB, shock, and hyperkalemia. Anti-hyperkalemic measures were taken at ICU with intravenous (i.v) regular insulin of 10 units with dextrose (50% of 50 mL) thrice daily, intravenous calcium gluconate (10% of 10 mL), salbutamol nebulization (5–10 mg) every 6 h, intravenous sodium bicarbonate (50–100 meq) along with oral sodium polystyrene sulfonate sachets (1 g) thrice daily. Diuretic (furosemide: 20 mg) was added for pleural effusion and hyperkalemia as well. AV nodal blockers (bisoprolol and amlodipine) were stopped. Hyperkalemia-causing medication (spironolactone and telmisartan) was also stopped. The renal function test was done in 12 h. The nephrology team was consulted regarding the potential need for hemodialysis if medical management fails. Cardiology consultation was sought for the probable need for a temporary pacemaker.
Further workup revealed her hs Troponin-I level to be normal. ECG confirmed valvular heart disease with aortic and mitral stenosis. The infectious work up (blood, urine, and sputum culture) was negative.
Despite initial interventions, her hyperkalemia persisted. The frequency of anti-kalemic measures was increased. Heart rate increased to 45–55/min. ECG changes continued to persist. Finally, a decision was made combinedly by the ICU team, nephrology team, and cardiology team to initiate hemodialysis for refractory hyperkalemia. A hemodialysis catheter was placed in the right jugular vein, and hemodialysis was started.
After 48 h of ICU admission, she was stable with a sinus rhythm (P-wave visible in ECG), HR of 80–90 beats per minute (Figure 2), and potassium of 4.4 mmol/L. Her antihypertensive medications were readjusted to prazosin 2.5 mg 12-hourly. Her antiplatelets and antidyslipidemic drugs were also resumed. AV nodal-blocking drugs (bisoprolol and amlodipine) were avoided.
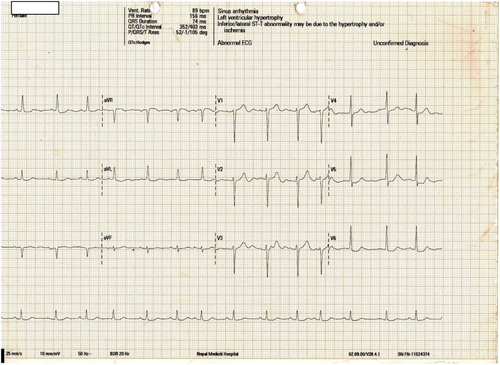
2.2 Outcome and Follow-Up
The patient was transferred to the ward after 4 days of ICU admission. Recommendations were made for cardiology and nephrology follow-up prior to discharge, along with a caution against the usage of AV nodal blockers (such as bisoprolol and amlodipine). Instead, it was advised to change her antihypertensive medications to alpha blocker (prazosin). The ECG conducted at the ward showed a normal sinus rhythm. Following a week-long hospital stay, the patient was discharged home (Table 1).
| Laboratory values | Emergency | ICU Day 1 | ICU Day 3 | ICU Day 4 | References |
|---|---|---|---|---|---|
| TLC | 10,730 | 8550 | 7310 | 8980 | 4000–11,000 Cells/Cumm |
| Hemoglobin | 10.6 | 8.8 | 8.5 | 9.2 | 11.9–14.6 g% |
| Platelets | 329,000 | 243,000 | 212,000 | 227,000 | 150,000–450,000 Cells/Cumm |
| PCV | 33 | 37.6 | 41.2 | 42.3 | 40%–50% |
| Creatinine | 1.4 | 1.6 | 1.3 | 1.0 | 0.52–1.04 mg/dL |
| Potassium | 7.5 | 6.8 | 4.4 | 4.0 | 3.5–5.1 mmol/L |
| Sodium | 134 | 136 | 138 | 140 | 137–145 mmol/L |
| Urea | 110 | 76 | 52 | 62 | 15–45 mg/dL |
| Total protein | 6.4 | N/A | N/A | 6.1 | 6.3–8.2 g/dL |
| Albumin | 3.5 | N/A | N/A | 3.2 | 3.5–5 g/dL |
| ALT/SGPT | 42 | N/A | N/A | 22 | < 35 U/L |
| AST/SGOT | 25 | N/A | N/A | 24 | 14–26 U/L |
| Total bilirubin | 0.3 | N/A | N/A | 0.4 | 0.2–1.3 mg/dL |
| ALP | 68 | N/A | N/A | 60 | 38–126 U/L |
| PT/INR | 13.6/1.05 | N/A | N/A | N/A | 11–16 s |
| CRP | 28.1 | N/A | N/A | 20.3 | < 10 mg/dL |
| CK-MB | 13 | N/A | N/A | N/A | 0–16 U/L |
| CPK | N/A | 55 | N/A | N/A | |
| LDH | N/A | 194 | N/A | N/A | |
| High-sensitive Troponin-I quantitative | 11.04 | N/A | N/A | N/A | < 9 ng/L |
| TSH | 7.07 | N/A | N/A | N/A | 0.46–4.68 uIU/mL |
| Calcium | 8.2 | 8 | N/A | N/A | 8.4–10.2 mg/dL |
| GRBS | 166 | 154 | 142 | 120 | 80–140 mg/dL |
3 Discussion
BRASH syndrome is a combination of impaired renal clearance that causes hyperkalemia and the build-up of AV nodal blocking medications, leading to bradycardia and shock [2]. While cases of refractory bradycardia despite correction of hyperkalemia have been described in the literature, BRASH syndrome has only been recently named by Josh Farkas MD, the first published case using the term BRASH Syndrome [2]. Patients with BRASH syndrome are frequently adherent to proper medication dosing and rarely have supra-therapeutic blood levels of AV nodal blocking agents [1]. Bradycardia subsequently results in decreased cardiac output, leading to diminished renal perfusion and subsequent development of acute kidney injury, along with exacerbation of hyperkalemia. Our patient, who was on beta blockers, landed at the emergency unit with bradycardia, shock, and hyperkalemia.
Fluid resuscitation and aggressive hyperkalemia therapy are the mainstays of treatment for moderate cases of BRASH syndrome. In contrast, mild cases often respond well to simpler interventions like intravenous calcium and fluid resuscitation [3].
Isolated hyperkalemia may lead to bradycardia, subsequently inducing renal failure, but typically, severe hyperkalemia (e.g., potassium over ∼7 mEq/L) is required to trigger bradycardia [4]. This distinction from BRASH syndrome, characterized by more moderate hyperkalemia, is crucial. An electrocardiogram showing bradycardia without other hyperkalemic features serves as a significant indicator of BRASH syndrome [5]. Similar findings were present in our study as well.
Rapid management of hyperkalemia entails administering IV calcium to stabilize the cardiac membrane and enhance cardiac output, intravenous insulin, and dextrose, as well as beta agonists to facilitate intracellular potassium shifting, sodium polystyrene sulfonate, and sodium zirconium cyclosilicate to lower total body potassium levels, with both agents binding potassium in the colon and promoting its excretion. Additionally, furosemide serves as a potassium dumping agent by facilitating renal potassium excretion. Renal replacement therapy, such as hemodialysis, may be required as a second line of treatment, especially in patients with end-stage renal disease.
Proper clinical history helps differentiate BRASH syndrome from intoxication with beta blockers or calcium channel blockers, which also leads to bradycardia and shock, as patients with BRASH syndrome typically take their medications as directed and do not generally involve supratherapeutic drug levels. Our patient was on the usual dosage regimen of beta blockers and had no history of overdosing.
Management of bradycardia begins with IV calcium to counteract hyperkalemia effects. If bradycardia persists, epinephrine infusion is recommended, as it increases heart rate, cardiac output, and shifts potassium intracellularly. Isoproterenol is an alternative for patients unresponsive to epinephrine. Standard advanced cardiac life support algorithms may inadequately address BRASH syndrome as they do not involve calcium administration, underscoring its clinical significance [1]. Despite maintaining normal blood pressure, some patients with severe bradycardia may experience malperfusion, requiring treatment to restore systemic perfusion and renal function, with isoproterenol or dobutamine considered as therapeutic options [1].
Hypovolemia, a common trigger, requires prompt treatment based on clinical history and bedside point of care ultrasonography (POCUS) examination. Isotonic bicarbonate is effective for patients with uremic acidosis and hyperkalemia, whereas balanced crystalloid solutions are preferred over normal saline for non-acidotic patients to avoid a potential rise in serum potassium.
Advanced therapies such as lipid emulsion, glucagon, or high-dose insulin infusion can be considered for reversing beta blocker or calcium channel blocker toxicity in patients with BRASH syndrome, particularly those taking renally cleared beta blockers. Patients on unusually large doses of multiple AV nodal blocking agents also benefit from these treatments. Digoxin-specific antibody fragments are recommended if digoxin toxicity is suspected. Adrenal insufficiency should be addressed with stress dose corticosteroids, typically hydrocortisone 100 mg i.v. Transvenous pacing can be considered as a salvage maneuver if medical management for bradycardia is ineffective.
Recognition and adjustment of the triggering agent are of utmost importance and should be done immediately. Patients may present with a variety of non-specific signs and symptoms including symptomatic bradycardia, syncope, generalized weakness, altered mental status, dyspnea or dizziness/lightheadedness, and others, which makes the diagnosis of BRASH syndrome very challenging [6]. Beta blocker was immediately put on hold and was switched to other anti-hypertensive agents for hypertension management in our study.
Older patients with multiple comorbidities, mainly cardiac and renal diseases, may be at higher risk of developing BRASH, especially if their medications include multiple different AV nodal blocking medications [1].
In our case, the clinical suspicion of BRASH syndrome was prompted by the identification of the aforementioned array of symptoms, excluding major differential diagnoses such as sepsis or acute cardiac conditions.
Collaboration with a multidisciplinary team comprising cardiology and nephrology specialists is crucial, with considerations for temporary pacemaker insertion and the potential need for hemodialysis; our patient required a single hemodialysis session to manage refractory hyperkalemia.
In some cases, cardiac resuscitation following the advanced cardiac life support (ACLS) protocols may not be successful. This could raise the impression that the vicious cycle of BRASH syndrome might be irreversible after it reaches a certain point [3].
4 Conclusion
The confluence of hyperkalemia, hypotension, and bradycardia in the setting of kidney dysfunction and medications that block the atrioventricular node could be entities of BRASH Syndrome. Hemodynamic support, renal replacement therapy, and a temporary pacemaker may be necessary to manage this fatal entity.
Author Contributions
Bikash Khadka: conceptualization, formal analysis, investigation, methodology, project administration, validation, writing – original draft, writing – review and editing. Saroj Poudel: data curation, project administration, resources, writing – review and editing. Kishor Khanal: formal analysis, methodology, supervision, writing – original draft, writing – review and editing. Anup Ghimire: methodology, writing – review and editing. Ashim Regmi: methodology, writing – review and editing. Sharad Shrestha: methodology, writing – review and editing. Shirish KC: formal analysis, writing – review and editing. Rohini Nepal: writing – review and editing.
Acknowledgments
We sincerely thank all healthcare members of our critical care department for their efforts.
For the ongoing mutual support for research capacity improvements, we would also like to acknowledge and thank members of the Doctors on Wheels:
Shirish KCb,d, Rohini Nepalb,c, Dinesh Duwalb,e, Vikram Tiwarib,f, Bikash Khadkaa, Priyesh KCb, Sinju Adhikarib,g, Smriti Maharjanb, Ritika Gurungb, Rashika Bisunkeb,g, Roshan Khadkab, Bijay Bikram Singhb, Rika Rijalg, and Pushpa Bahadur KCb
aDepartment of Anaesthesiology and Critical Care, Nepal Mediciti, Nepal
bDepartment of Clinical Research, Doctors on Wheels, Kathmandu, Nepal
cDepartment of Internal Medicine, NYC Health and Hospitals/Woodhull, New York, USA
dDepartment of Neurology, Columbia University Irving Medical Center, New York, USA
eDepartment of Ophthalmology, Tribhuwan University Teaching Hospital, Nepal
fChapakot Hospital, Syangja, Nepal
gRhythm Neuropsychiatry Hospital and Research Center, Nepal
Ethics Statement
This case report did not require the approval of any Ethical Committee.
Consent
Written informed consent was obtained from the patient and patient party.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




