The significance of therapeutic drug monitoring in detecting low medication adherence in patients with cancer: A case study of cabozantinib
Key Clinical Message
Maintaining good medication adherence is important for providing desirable outcomes from medication therapy. We showed that therapeutic drug monitoring (TDM) contributed to the identification of low medication adherence to cabozantinib in a patient with cancer. We present an educational case to assist with understanding TDM in a patient with cancer.
1 INTRODUCTION
Remarkable advancements have occurred in oral drug development for cancer in recent years, which has allowed patients to opt for outpatient treatments. In particular, the development of small molecule inhibitors, such as tyrosine kinase inhibitors (TKIs), has expanded rapidly since the early 2000s, with 88 small molecule inhibitors having been approved by the US FDA for oncology indications by 2022.1 However, several considerations should be taken into account regarding the pharmacokinetics of these drugs, including possible (1) significant reduction in therapeutic efficacy due to interactions during the absorption process, such as changes in gastric pH and complex formation with food and drink components,2-4 (2) increased toxicity due to interactions with therapeutic drugs for chronic diseases, especially via the metabolic pathway mediated by cytochrome P450 (CYP),5 and (3) significant fluctuations in absorption due to meals high in fat.3 One strategy to solve these complex pharmacokinetics is the use of therapeutic drug monitoring (TDM), which involves dose adjustment based on blood concentrations. TDM can adjust drug doses based on individual patients and optimize drug concentrations. Moreover, it is already used in other therapeutic areas, such as antimicrobials and antiepileptic drugs, to ensure efficacy and safety. However, TDM of TKIs is not necessarily conducted. Whether patients take their TKIs properly relies on the healthcare providers' skills, such as history taking and confirming medication adherence. This could potentially impact the prognosis, resulting, for example, in cancer progression in the worst-case scenario. Here, we report a case in which TDM used in a patient with renal cell carcinoma receiving cabozantinib, a TKI, helped to identify their low medication adherence.
This study received ethical approval from The Ethics Committee of Saiseikai Yokohamashi Tobu Hospital (No. 20210229) and written informed consent was obtained from the patient.
2 CASE HISTORY
An 84-year-old male with renal cell carcinoma diagnosed 10 years earlier received four courses of nivolumab (240 mg/body) and ipilimumab (48 mg/body) and 17 courses of nivolumab (240 mg/body) as maintenance therapy at Saiseikai Yokohamashi Tobu Hospital, due to lung and bone metastases. However, due to tumor progression, we opted to switch the treatment to cabozantinib, which inhibits multiple receptor tyrosine kinases, including mesenchymal-epithelial transition factor, vascular endothelial growth factor receptor 2, and AXL. Given the patient's advanced age and the anticipated risk of severe adverse events, the starting dose of cabozantinib was set at 40 mg/day.
3 METHODS
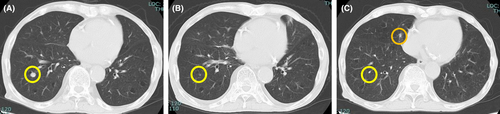
On day 14, the cabozantinib concentration determined using a high-performance liquid chromatography with ultraviolet detector (HPLC-UV)6 was 1121 ng/mL. At that time, grade 2 proteinuria and creatinine increase were observed, according to the Common Terminology Criteria for Adverse Events version 5.0.7 We therefore reduced the cabozantinib dose to 20 mg/day. After dose reduction, the cabozantinib concentration decreased to 697.1 ng/mL, and thereafter, the blood concentration of cabozantinib ranged from 565.8 to 780.0 ng/mL (days 63–182). Although grade 2 proteinuria persisted, renal function remained stable, and the adverse events were manageable. Therefore, cabozantinib treatment was continued. On day 118, treatment response was evaluated using computed tomography (CT), which showed a partial response (PR) according to Response Evaluation Criteria in Solid Tumors version 1.1;8 therefore, cabozantinib 20 mg was continued. On day 210, the cabozantinib concentration decreased to 337.9 ng/mL, and it was revealed that the patient was only taking cabozantinib approximately once every 2 days. Despite instructing the patient about the significance and importance of medication adherence, the cabozantinib concentration further decreased to 305.2 ng/mL on day 238. Evaluation of the tumor by CT showed slight enlargement of the lung metastases (Figure 1), but the condition was classified as a stable disease; therefore, cabozantinib was continued.
4 RESULTS
Upon further discussion with the patient, it was noted that the decreased medication adherence was because of taking the medication approximately 2 h after breakfast; hence, the timing was changed to immediately after waking up. On day 274, medication adherence improved and the blood concentration of cabozantinib increased to 618.3 ng/mL (Figure 2). Unfortunately, at approximately 274 days, along with an increase in cabozantinib concentration, a grade 3 rash occurred, which led to cabozantinib discontinuation. Seventy days after discontinuation of cabozantinib, the rash completely recovered, and we switched to axitinib, which does not interact with food, considering medication compliance and therapeutic effect.9, 10
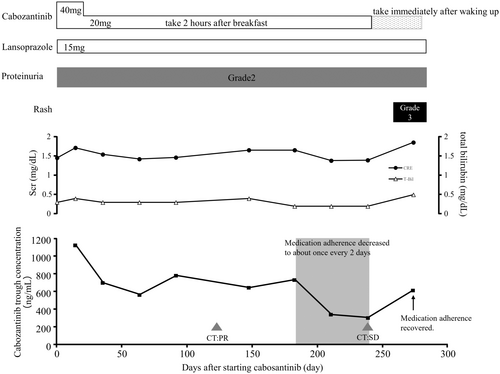
During the treatment period, the patient was taking clopidogrel, rosuvastatin, lansoprazole, olmesartan, amlodipine, febuxostat, and sitagliptin for a history of angina, hypertension, hyperuricemia, and diabetes, and the prescription of these drugs did not change throughout treatment. In addition, patients did not take any herbal or nutritional/vitamin supplements throughout the treatment period.
5 DISCUSSION
In this case report, we identified low cabozantinib adherence using TDM. Cerbone et al. reported that the anticancer effects of cabozantinib require a trough concentration of over 536.8 ng/mL.11 In our patient, cabozantinib trough concentrations ranged from 643 to 780 ng/mL during adequate adherence, and a PR was observed. In contrast, the tumor increased slightly during low medication adherence, when the trough concentrations ranged from 305.2 to 337.9 ng/mL. This finding partially supports the suitable trough concentration higher than 536.8 ng/mL suggested by Cerbone et al.
The background of poor medication adherence is somewhat complex, predominantly involving three scenarios: (1) forgetting or misunderstanding the correct administration of medications, (2) challenges in medication intake due to factors, such as large pill size or inconvenient timing, and (3) deliberately pretending to do it out of goodwill towards healthcare providers. Above mentioned poor medication adherence can be confirmed through interviews, assessing leftover medicine, or identifying undesirable symptoms related to the disease.12 Unfortunately, the practice of determining low medication adherence by these means depends on healthcare providers' skills. TDM can objectively demonstrate drug–drug or food-drug interactions and assess low medical adherence. For example, drug-beverage interactions involving various combinations of fruit juice or green tea significantly alter drug concentrations.13-16 This issue is critical as patients often remain unaware of these interactions. Besides, in drug–drug interactions, TDM reportedly identified that prednisolone stimulates sorafenib metabolism.17 In medication adherence, TDM reportedly identified low medication adherence in patients treated with azathioprine, anti-HIV drugs, and antihypertensive drugs.18-20 However, additional reports of TDM contributing to the improvement of low medication adherence have not been sufficiently disclosed before. In general, the purposes of TDM are to (1) maintain an effective drug blood concentration, (2) confirm medication adherence, and (3) avoid adverse effects.21 For example, TDM is often performed for antibiotics, with the aim of achieving prompt therapeutic effects over a short term, while avoiding long-term adverse effects. For antiarrhythmic or antiepileptic drugs, the goal is often to manage symptoms and avoid adverse effects. TDM is particularly effective for drugs with large inter-individual variation. In addition to these drugs, oral TKIs are known to exhibit significant inter-individual variation, as exemplified by differences in drug-metabolizing enzymes and drug efflux transporters.22-24
Recently, several TKIs have been reported to exhibit a relationship between therapeutic efficacy and blood concentration.25-27 The lifespan in patients with cancer is increasing with the development of novel TKIs. This suggests that TDM of TKIs is increasingly important in patients with cancer who undergo long-term treatment, not only for anticancer effects, but also to avoid adverse effects due to either excess blood concentration or of blood concentrations dropping due to low medication adherence.
6 CONCLUSION
This case report highlights the importance of the early detection of decreased medication adherence through regular cabozantinib TDM. We emphasize that TDM of TKIs may be important in identifying low medication adherence, even if there is a lack of evidence for the relationship between anticancer effects and anticancer drug blood concentration.
AUTHOR CONTRIBUTIONS
Shinichi Maruyama: Conceptualization; formal analysis; investigation; methodology; project administration; validation; visualization; writing – original draft. Kenji Momo: Conceptualization; formal analysis; methodology; project administration; validation; visualization; writing – original draft. Tadatsugu Anno: Conceptualization; investigation; methodology; project administration; resources; supervision; writing – review and editing. Masaru Ishida: Methodology; project administration; resources; supervision; writing – review and editing. Hiroshi Kanno: Project administration; supervision; writing – review and editing. Masaru Kato: Funding acquisition; project administration; resources; supervision; writing – review and editing.
FUNDING INFORMATION
This work was supported in part by the JSPS KAKENHI (M Kato, grant number: 18H02560). We thank the clinical laboratory staff who collected the blood samples at Saiseikai Yokohamashi Tobu Hospital. We also thank Editage (www.editage.jp) for English language editing.
CONFLICT OF INTEREST STATEMENT
The Department of Hospital Pharmaceutics, School of Pharmacy, Showa University, received funds from Ono for a contract research project in accordance with a collaborative research agreement. As potential conflicts of interest related to the publication of this study, Hospital Pharmaceutics received research grants from Daiichi Sankyo, Mochida, Shionogi, Ono, Taiho, Nippon-Kayaku, and Bayer. KM received honorariums from Abbvie, Eisai, Sawai, and Nippon-Kayaku for his presentations. The other authors declare no conflicts of interest associated with this manuscript.
ETHICS STATEMENT
This study received ethical approval from The Ethics Committee of Saiseikai Yokohamashi Tobu Hospital (No. 20210229).
CONSENT
We obtained written informed consent for publication from the patient.
Open Research
DATA AVAILABILITY STATEMENT
All information in this case is included in this published article.




