Low cholesterol levels are good markers for central hypothyroidism in case with dialysis using roxadustat
Key Clinical Message
Recently, hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitors have been used for renal anemia, but side effects have also been reported. We report on the association of central hypothyroidism and cholesterol with roxadustat. Based on this case and previous reports, we believe that patients receiving roxadustat should have their thyroid function and cholesterol levels checked regularly.
1 INTRODUCTION
Renal anemia is an avoidable and critical complication in cases of chronic renal failure. The prevalence of anemia in Japan was 7.8% in CKD stage G3a, 18.1% in G3b, 40.1% in G4, and 60.3% in G5; erythropoiesis-stimulating agent (ESA) use was 7.9% in CKD stage G4 and 22.4% in CKD stage G5.1 For a long time, treatment of renal anemia was centered on replacement therapy with erythropoiesis-stimulating agent (ESA) and iron supplements. However, thrombotic and embolic complications are peculated as side effects of HIF-PHIs.
In recent years, hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitors, which are orally administrable drugs for treating renal anemia, have been used.2 HIF-PH inhibitors activate hypoxia-inducible factor (HIF) in the body to stimulate the production of erythropoietin (EPO) and increase hemoglobin levels. Its effectiveness in erythropoiesis is comparable to that of ESA, and it is characterized by its oral rather than injectable formulation, its mechanism of promoting iron absorption from the intestinal tract and iron utilization in the body, and its ability to increase hemoglobin at physiological EPO blood levels.
However, along with the many benefits, cautions have also been pointed out when using HIF-PH inhibitors. There are some concerns about its effects on cancer cells and retinal hemorrhage.3-7 Roxadustat is an oral HIF-PH inhibitor that stimulates erythropoiesis. Here, we showed a case of central hypothyroidism caused by roxadustat with a summary of our similar cases. To find out central hypothyroidism, low cholesterol levels were good markers.
2 CASE HISTORY/EXAMINATION
The patient, a 69-year-old man, was diagnosed with hyperglycemia beginning in 1998 and started treatment in 2001 with a diagnosis of type 2 diabetes mellitus. He started hemodialysis in 2017 due to end-stage renal failure with diabetes-related kidney disease as the primary disease. Roxadustat (a HIF-PH inhibitor) was started at a dose of 100 mg (1.47 mg/kg) for recombinant human EPO-resistant anemia in August 2020. The dose of roxadustat was increased to 200 mg (2.94 mg/kg) to improve his anemia. TSH and fT4 were within the normal range during recombinant human EPO administration. General malaise gradually developed after starting roxadustat. His TSH and fT4 gradually decreased to 0.47 μIU/mL and 0.15 ng/dL, respectively. Furthermore, cholesterol levels were markedly low (Total-C 84 mg/dL, HDL-C 48 mg/dL, LDL-C 31 mg/dL). Levothyroxine (a synthetic thyroxine) was started at a dose of 12.5 μg for hypothyroidism in February 2021 and was admitted to the hospital for further examination. Oral medications at an administration were levothyroxine 12.5 μg, aspirin 100 mg, clopidogrel sulfate 75 mg, vonoprazan fumarate 10 mg, febuxostat 20 mg, alfacalcidol 0.5 μg, linagliptin 5 mg, valsartan 160 mg, nifedipine 80 mg, and sucroferric oxyhydroxide 750 mg. He is 168 cm tall and weighs 68 kg. There was no fever or hypothermia, blood pressure was 160/84 mmHg, and pulse rate was 78 beats/minus. There were no abnormal physical findings, including thyroid, and no abnormal body hair or decreased sweating.
3 METHODS
Laboratory data is shown in Table 1. Thyroid-related auto-antibodies were negative. No abnormalities were observed in other pituitary hormones. Brain MRI revealed no abnormality in the pituitary gland. A TRH stress test (500 μg of synthetic TRH was injected intravenously and TSH and fT3 and fT4 were measured.) was performed and the patient was diagnosed with central hypothyroidism (Table 2).
| RBC (470 × 104–610 × 104/μL) | 367 × 104 | AST (13–33 U/L) | 13 |
| Hb (14.0–18.0 g/dL) | 11.5 | ALT (8–42 U/L) | 8 |
| MCV (80–99 fL) | 101 | ALP (38–113 U/L) | 420 |
| Ret (0.3%–2.0%) | 1.7 | γ-GTP (10–47 U/L) | 11 |
| WBC (4800–10800/μL) | 5000 | BUN (8–22 mg/dL) | 64 |
| Plt (130 × 103–400 × 103/μL) | 194000 | Cr (0.6–1.00 mg/dL) | 8.54 |
| CRP (−0.30 mg/dL) | 2.61 | LDH (119–229 U/L) | 168 |
| Na (135–149 mEq/L) | 135 | T-Cho (128–219 mg/dL) | 84 |
| Cl (96–108 mEq/L) | 105 | HDL-Cho (40–99 mg/dL) | 48 |
| K (3.5–4.9 mEq/L) | 7.2 | LDL-Cho (70–139 mg/dL) | 31 |
| Ca (8.0–10.5 mg/dL) | 8.4 | non-HDL (−169 mg/dL) | 36 |
| P (2.5–4.5 mg/dL) | 5.2 | Glu (69–109 mg/dL) | 138 |
| UA (3.6–7.0 mg/dL) | 2.4 | HbA1c (4.6%–6.2%) | 4.9 |
| TP (6.7–8.3 g/dL) | 5.9 | BNP (−18.4 pg/mL) | 392 |
| Alb (4.0–5.0 g/dL) | 2.5 | TSH (0.61–4.23 μIU/mL) | 0.47 |
| T-Bil (0.4–1.2 mg/dL) | 0.3 | FT3 (2.51–4.16 pg/mL) | 0.70 |
| CK (62–287 U/L) | 59 | FT4 (0.83–1.77 ng/dL) | 0.15 |
- Abbreviations: FT3, Free triiodothyronine; FT4, Free thyroxine; TSH thyroid stimulating hormone.,
| TSH | fT3 | fT4 | |
|---|---|---|---|
| TRH Test during roxadustat taking | |||
| Pre | 0.18 | 0.87 | 0.15 |
| 15 min | 0.56 | ||
| 30 min | 0.73 | ||
| 60 min | 0.75 | ||
| 90 min | 0.77 | ||
| 120 min | 0.48 | 0.82 | 0.23 |
| TRH test after completion of roxadustat therapy | |||
| Pre | 4.74 | 1.94 | 0.84 |
| 15 min | 10.75 | ||
| 30 min | 15.45 | ||
| 60 min | 16.08 | ||
| 90 min | 16.28 | ||
| 120 min | 14.90 | 2.09 | 0.86 |
| Thyroid related auto-antibody | |
|---|---|
| TSH receptor Ab | 0.8 IU/L |
| Anti-TPO Ab | 12.64 IU/mL |
| Anti-thyrogloblin Ab | 14.18 IU/mL |
| Other hormones | |
|---|---|
| ADH | 4.4 pg/mL |
| ACTH | 61.5 pg/mL |
| GH | 4.5 ng/mL |
| Somatomedin C | 77 ng/mL |
| LH | 9.44 mIU/mL |
| FSH | 13.04 mIU/mL |
| PRL | 31.4 ng/mL |
| IRI | 1.81 μIU/mL |
| Cortisol | 10.9 μg/dL |
| Free testosterone | 8.6 pg/mL |
- Abbreviations: ADH, antidiuretic hormone; FT3, Free triiodothyronine; FT4, Free thyroxine; GH, growth hormone; IRI, immunoreactive insulin; LH, luteinizing hormone; PRL, prolactin; TPO, thyroid peroxidase; TRH, thyrotropin-releasing hormone; TSH, thyroid stimulating hormone.
4 CONCLUSION AND RESULTS
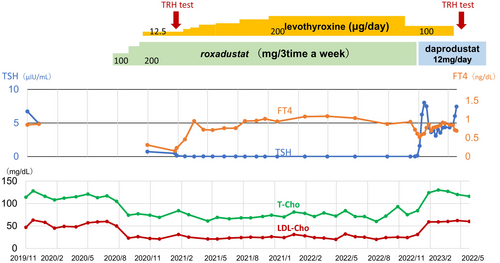
The dose of levothyroxine was increased for central hypothyroidism. After administration of 200 μg of levothyroxine, fT4 recovered quickly into the normal range and general malaise improved, but TSH did not recover. Central hypothyroidism might be speculated as an adverse effect of roxadustat. In January 2021, roxadustat was switched to daprodustat (Figure 1). After discontinuing roxadustat, TSH recovered rapidly into the normal range. Moreover, cholesterol levels increased. TRH test was performed again, and the results were normal. Therefore, central hypothyroidism was reversible. Levothyroxine was tapered and discontinued, but thyroid function remained within the normal range.
5 DISCUSSION
We present a case of a dialysis patient who developed central hypothyroidism after using roxadustat. Table 3 is a summary of central hypothyroidism dialysis patients taking roxadustat at our hospital, all 7 of whom were hemodialysis patients. All cases decreased TSH and fT4 after taking roxadustat. Roxadustat was discontinued in 7 patients, and the dose was reduced in 1 patient, TSH and fT4 recovered. Although central hypothyroidism by roxadustat would be reversible. Therefore, early detection of central hypothyroidism is required.
| Central hypothyroidism | Levothyroxine | Maximum dose of Roxadustat (mg) (mg/kg) | Duration of Roxadustat (day) | Change in total cholesterol levels(mg/dL) | Supplement | |
|---|---|---|---|---|---|---|
| (1) 69 M | (+) | (+) | 200 (2.89) | 906 | 105 → 74 (−31) | Decreased to below TSH detection sensitivity |
| (2) 72 M | (+) | (−) | 100 (1.64) | 570 | 171 → 114 (−55) |
Only at high doses of roxadustat Temporary TSH suppression |
| (3) 75 M | (+) | (+) | 200 (3.60) | 276 | 114 → 72 (−42) | Decreased to below TSH detection sensitivity |
| (4) 70 F | (+) | (+) | 70 (2.10) | 415 | 192 → 85 (−107) | Dose adjustment of roxadustat caused thyroid hormone fluctuations |
| (5) 75 M | (+) | (+) | 120 (1.30) | 499 | 116 → 86 (−30) | |
| (6) 69 M | (+) | (+) | 120 (2.10) | 204 | 152 → 113 (−39) | |
| (7) 85 F | (+) | (+) | 40 (1.12) | 315 | 115 → 76 (−39) |
Onset of central hypothyroidism at low doses History of subclinical thyrotoxicosis |
| (8) 59 M | (+) | (−) | 70 (1.56) | 52 | 194 → 113 (−81) |
No new cholesterol-lowering medications were started in any patient after initiation of roxadustat. Markedly reduced cholesterol levels would be good markers to speculate hypothyroidism. The decreased cholesterol levels were recovered in all cases after discontinuing or reducing roxadustat in a similar way to recover thyroid function.
The central hypothyroidism in our case was reversible. And the central hypothyroidism would depend on taking roxadustat. Similar cases were reported previously.3, 5 Some drugs, such as retinoid X receptor selective ligands, have been reported to suppress TSH gene promoter activity, thereby causing central hypothyroidism.8 Therefore, it is speculated that roxadustat caused central hypothyroidism by a similar mechanism. Another possibility is the direct effect on the thyroid hormone receptor. Roxadustat has a similar chemical structure to T3. It would have a stronger affinity for the thyroid hormone receptor TRβ than T3.9 Thereby, it acts as an agonist on the thyroid hormone receptor TRβ present in the hypothalamus and pituitary gland, suppressing TSH secretion. Additionally, it has been reported that roxadustat may partially cross the blood–brain barrier in mice.10 Therefore, it is possible that orally administered roxadustat crosses the blood–brain barrier, and binds to thyroid hormone receptor TRβ. These are possible mechanisms of central hypothyroidism after using roxadustat.
In our case, cholesterol levels were markedly reduced in the condition of central hypothyroidism. Thyroid hormones have important effects on cell development, growth, and metabolism, and are expressed and act in almost all tissues.11, 12 Thyroid hormone binds to thyroid hormone receptors in the nucleus and lowers cholesterol levels through TRβ. TRβ stimulation increases LDL receptor expression in the liver, resulting in plasma clearance of LDL cholesterol.13 As described previously, it is speculated that roxadustat binds to TRβ,9 increases the expression of LDL receptors, and lowers cholesterol. Therefore, it is speculated that reduced cholesterol levels are an agonistic effect of roxadustat on the TRβ1 receptor in the liver. All of the seven cases, including the present case, showed significant cholesterol reduction during treatment with roxadustat, which improved after discontinuation or reduction of the drug. Hypocholesterolemia is presumed to be a TRβ-mediated effect similar to the TSH secretion effect of roxadustat in the brain, so the detection of hypocholesterolemia is an important marker for finding central hypothyroidism. However, these are only speculative potential mechanisms for the development of central hypothyroidism and hypocholesterolemia associated with roxadustat, and further studies are required.
In addition to central hypothyroidism, HIF-PH inhibitors have been suggested to be associated with various adverse events; such as thromboembolism, malignancy, and retinopathy. Since the risk of thromboembolism is higher in patients with a history of thromboembolism, it is recommended that HIF-PH inhibitors be carefully considered. Sudden increases in hemoglobin levels and iron deficiency also increase the risk of thromboembolism and should be treated with caution. Although there is no evidence that HIF-PH inhibitors increase the risk of cancer, activation of HIF by HIF-PH inhibitors may promote growth of cancer cells and/or metastasis. Close examination of cancer before and after HIF-PH inhibitor administration is recommended.14 In addition, HIF-PH inhibitors may increase the expression of VEGF, retinal hemorrhage should be checked in patient with diabetic retinopathy.6
We experienced a dialysis case with hormonal dynamics similar to central hypothyroidism caused by roxadustat. A decrease in cholesterol was observed during the administration of roxadustat, and the patient developed symptoms after discontinuing roxadustat. The TRH stress test after discontinuing the drug showed a normal response, and this condition was judged to be a reversible change. It was speculated that roxadustat acts suppressively as an agonist in the hypothalamus and pituitary gland, and as an agonist in target organs.
AUTHOR CONTRIBUTIONS
Serina Kita: Conceptualization; data curation; formal analysis; investigation; project administration; resources; software; writing – original draft. Hiroshi Okuyama: Conceptualization; data curation; formal analysis; investigation; supervision. Takaya Kondo: Data curation; supervision. Mizuki Hayashi: Data curation; supervision. Shinichiro Nakao: Data curation; supervision. Toshitaka Sawamura: Data curation; formal analysis; supervision. Keiji Fujimoto: Data curation; formal analysis; methodology; project administration; supervision. Atsushi Nakagawa: Supervision. Hitoshi Yokoyama: Supervision; validation. Kengo Furuichi: Conceptualization; data curation; formal analysis; methodology; project administration; software; supervision.
ACKNOWLEDGMENTS
We thank the patient and his family for their cooperation.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors state that the study was conducted without any commercial or financial relationships that could be interpreted as a conflict of interest.
ETHICS STATEMENT
All procedures performed in studies involving human participants were by the ethical standards of the institutional committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
CLINICAL TRIAL REGISTRATION
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data were created or analyzed in this study.




