Symptomatic right side extralobular pulmonary sequestration in a young woman: A rare case report
Key Clinical Message
Pulmonary sequestration is uncommon congenital malformation. It is important to consider pulmonary sequestration even in young adults presenting with recurrent pneumonic symptoms because prompt surgical intervention is both feasible and curative. Moreover, in health settings lacking CT/MRI angiography service, pulmonary sequestration can be easily misdiagnosed as smear negative pulmonary tuberculosis. Therefore, strong clinical suspicion is required to prevent patient mismanagement.
1 INTRODUCTION
Pulmonary sequestration also known as bronchopulmonary sequestration is described as an abnormal lung tissue which has no connection with the tracheobronchial tree. In addition, this dysplastic lung tissue is nonfunctional, and receives blood supply via aberrant systemic artery.1 Pulmonary sequestration was first reported by Rokitanski and Rektorzik in 1861, and comprises only 0.15%–6.4% of all congenital lung anomalies.2, 3 There are at least four sub types, and these are: Intralobular sequestration (ILS), Extralobular sequestration (ELS), Hybrid Bronchopulmonary sequestration/Congenital pulmonary airway malformation (CPAM) lesions, and Bronchopulmonary foregut malformation.4 ILS resides with in the normal lung tissue, and has no covering pleura separating it from the surrounding functional lung tissue. On the other hand, ELS has covering visceral pleura, and is completely separated from the normal lung tissue. Moreover, the ILS is by far the commonest sub-type accounting for 75%–90% of pulmonary sequestrations followed by ELS which is seen in 10%–25% of the cases.5 Majority of pulmonary sequestrations are symptomatic usually presenting with recurrent pneumonia; however, some pulmonary sequestrations can be asymptomatic only diagnosed incidentally during imaging studies.6 We present a case of right side extralobular pulmonary sequestration in a 25-year-old female patient, with a review of clinic histopathologic presentations, diagnosis, and management options of pulmonary sequestrations.
2 CASE HISTORY
A 25-year-old Ethiopian female patient presented complaining of productive cough of whitish sputum and pleuritic type chest pain of 2 weeks duration. At the onset her current illness, the patient also had low-grade fever, loss of appetite, and generalized body fatigue. Apart from that, the patient had no history of weight loss, night time sweating or contact with TB diagnosed patient. For these mentioned symptoms, the patient visited the nearby local primary hospital. At the primary hospital, she was investigated with laboratory tests which showed leukocytosis with left shift (on CBC) and raised ESR (Table 1); however, chest X-ray was not done due to unavailability in the primary hospital at that time. She was then treated with antibiotics (Ceftriaxone 1 g IV BID for 7 days and Azithromycin 500 mg po daily for 3 days) as in patient. After commencement of antibiotics, fever, body fatigue, and appetite loss had improved. Nevertheless, productive cough and chest pain had persisted despite full course of antibiotics. Therefore, the patient was referred to our teaching hospital for further investigation and assessment. During evaluation at our outpatient chest clinic, she reported experiencing recurrent bouts of similar symptoms (four times with in the last 10 months). She had also visited local health facilities during each episode, and had been prescribed varies antibiotics, which were effective in treating her symptoms until the last bout. However, the patient never had chest imaging before due to poor access and financial constraint. Otherwise, the patient has no known family history of asthma, diabetes mellitus, or cancer. She is nonsmoker and nondrinker.
| Name of the test | Result at the onset of symptoms before antibiotics | Result on 16th day from the onset of symptoms (post antibiotics treatment) |
|---|---|---|
| CBC | 13,500/mm3 with granulocyte percentage of 84% (elevated) | 8100/mm3 with granulocyte percentage of 51% (normal) |
| Blood film for malaria | Negative | N/D |
| ESR | 40 mm/h (elevated) | 5 mm/h (normal) |
| CRP | N/A | N/D |
| RBS (from capillary blood) | 152 mg/dL (normal) | 145 mg/dL(normal) |
| Serum BUN | N/A | 15 mg/dL (normal) |
| Sputum AFB | Negative 3 times | Negative 3 times |
| Realtime automated nucleic acid amplification (Xpert MTB/RIF assay) of sputum | N/A | Negative (mycobacterium tuberculosis not detected) |
| Rapid HIV antibody test | Negative | Negative |
- Abbreviations: AFB, acid fast bacilli; BUN, blood urea nitrogen; CBC, complete blood count; CRP, c-reactive protein; N/A, not available; N/D, available but not done;RBS, random blood sugar.
3 METHOD
Vital signs showed blood pressure 110/70 mm hg, pulse rate 78 beats per minute and regular, respiratory rate 18 breath per minute, temperature 36.2°C, and oxygen saturation 96% at atmospheric air. Chest was clear on auscultation. On posterior–anterior chest X-ray (taken on the 16th day from onset of symptoms), right lower lobe consolidation was seen (Figure 1). Moreover, laboratory tests were done to rule out infectious causes of lung consolidation; however, the results were non revealing (Table 1). The normal WBC count on CBC and normalized ESR (Table 1 right side) were both attributed to antibiotics treatment. Apart from that, the negative result from the tests for tuberculosis (sputum AFB and Xpert MTB/RIF assay) helped us to consider other differential diagnosis such as smear negative pulmonary tuberculosis, lung abscess/empyema, fungal pneumonia, noninfectious pathologies etc. However, fungal antibody tests were not available in our set up. Therefore, we opted for chest CT scan in order to have better parenchymal and vascular visualization. Accordingly, contrast enhanced chest CT (done on the 19th day from onset of symptoms) demonstrated 7.5 cm × 7.4 cm × 3 cm well defined multi loculated predominantly cystic mass with fluid level and thick enhancing wall located at the right basal posterior segment in the subpleural region Figures 2,3, and 4. The mass has separate arterial supply arising from the thoracic aorta and venous drainage to the branches of pulmonary vein (Figure 5). In addition, the mass forms an acute angle to the adjacent pleural outline, which suggests that it is extra-lobular intrathoracic mass (Figure 6). Lastly, echocardiogram, abdominal ultrasound and electrocardiogram demonstrated no other abnormalities.
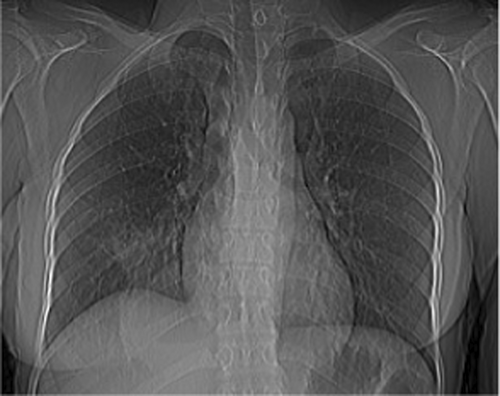
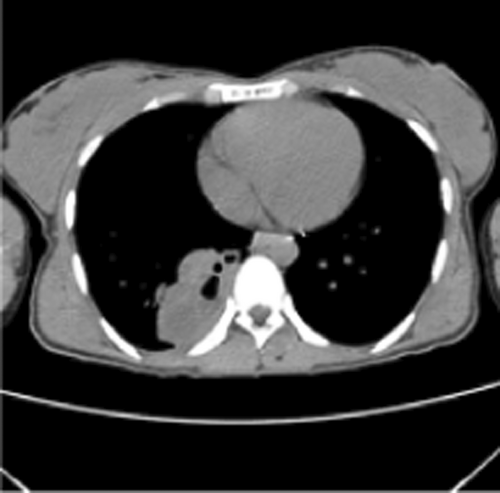
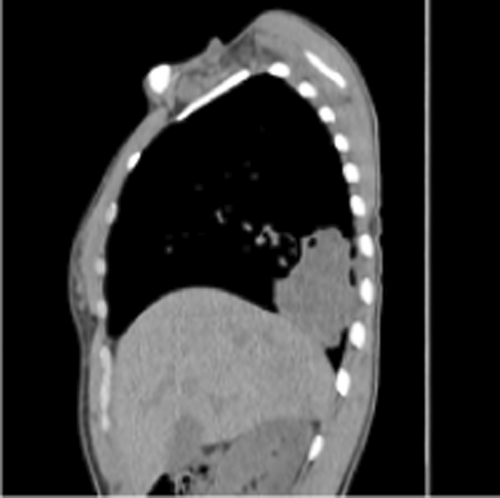
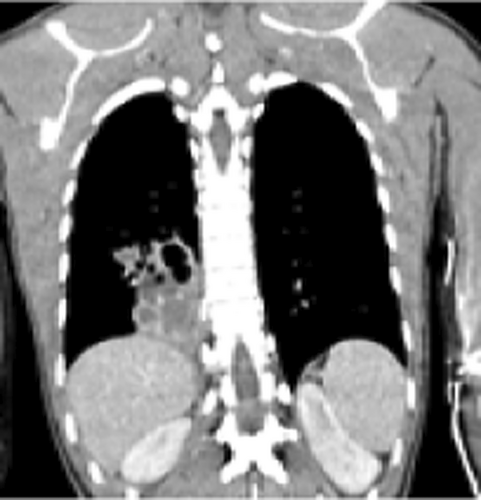
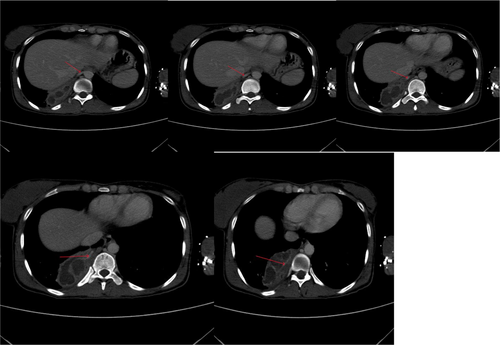

Hence, our presumptive diagnosis became infected extralobular pulmonary sequestration with abscess collection. In the meantime, given the recurrence of our patient's symptoms, and taking into account the future risk of malignant transformation, surgery was opted.
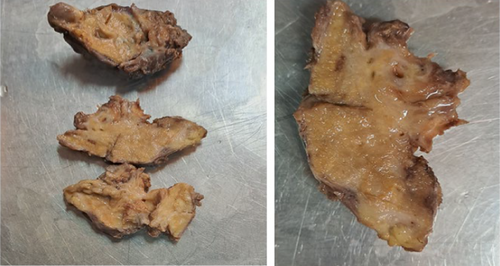
Subsequently, the patient was hospitalized for elective surgery, and right posterolateral thoracotomy was done on 24th day from onset of symptoms. Intraoperatively, there were multiple cavitary lesions in the right basal lung area which were interconnected by thick septae forming the mass. In addition, the mass was separated from the right lower lobe with a dense adhesion to the right hemidiaphragm and chest wall (indicates extralobular nature), and had 3.5 cm long arterial feeding vessel directly emerging from the descending thoracic aorta. The mass was resected uneventfully and sent for histopathologic assessment (Figure 7). Microscopic evaluation revealed few cystic dilated airways filled with secretary material and chronic inflammatory cells, and were embedded with in fibrotic stroma (Figures 8, 9, 10, and 11).
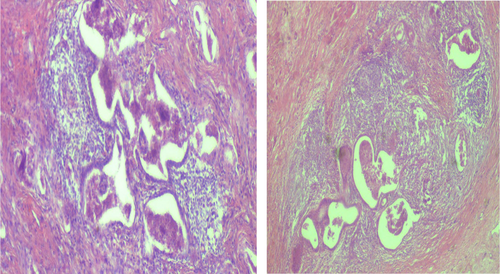
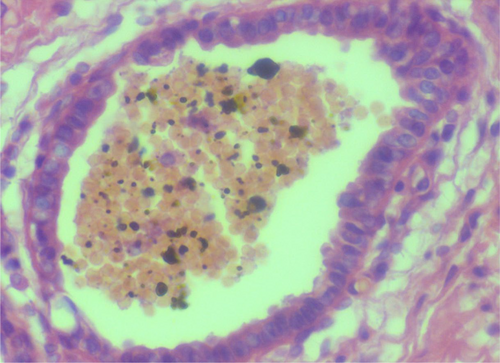
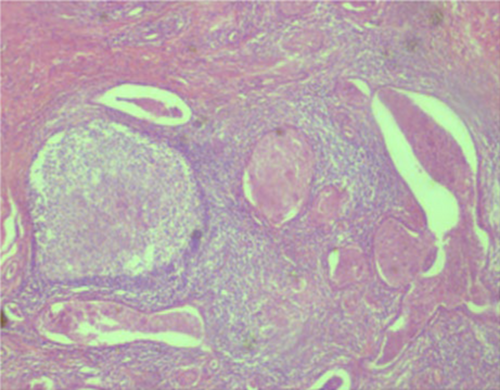
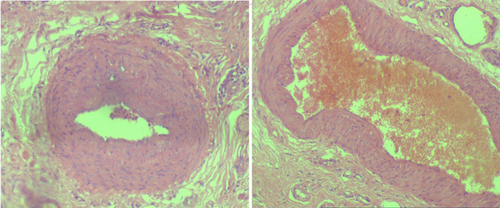
4 OUTCOME AND FOLLOW-UP
Histopathologic examination confirmed the diagnosis of extralobular pulmonary sequestration. The postoperative recovery was smooth, and she was discharged on the 4th post op day. Moreover, the patient was clinically followed for the subsequent 3 months and she showed complete resolution of symptoms with no recurrence.
5 DISCUSSION
Pulmonary sequestration is uncommon lower respiratory tract condition with poorly delineated embryologic mechanism. From its discovery till now, many theories have been proposed to elucidate the pathogenesis of pulmonary sequestration. The “Fraction Theory” proposed by Rokitanski and Rektorzik described pulmonary sequestration as separation of part of normally growing lung which subsequently turned-out to be nonfunctional.3, 7 The second hypothesis states that a damage to pulmonary arterial supply of portion of the lung during pulmonary embryogenesis favoring the proliferation of collateral artery from systemic circulation to supply that part of the lung (sequestered segment).8 Finally, the widely accepted theory elaborates that pulmonary sequestration occurs due to the formation of an ectopic lung bud inferior to the normally located lung buds that continued to migrate inferiorly with the foregut acquiring blood supply from systemic circulation (foregut vasculature).9
Pulmonary sequestration is generally assumed to be a childhood illness as the majority of patients are diagnosed during infancy. Nevertheless, both ILS and ELS can be diagnosed during adulthood.6, 10 ILS affects males and females equally, whereas ELS is more frequently seen in males.4 Regarding lobar distribution, more than 97% of pulmonary sequestrations were identified in the lower lobe. Apart from that, left lower lobe pulmonary sequestrations are 2–3 times commoner than right lower lobe lesions.2
Symptomatic pulmonary sequestrations usually present with recurrent pneumonia. There are also reported pulmonary sequestration cases presenting with severe shortness of breath, pleuritic chest pain, and hemoptysis.6 Although it is quite common to see asymptomatic adult ELS cases, our patient had history of recurrent respiratory symptoms with good response to antibiotics indicating recurrent pneumonitis. In her last episode however, she failed to respond to the pneumonia treatment. That could be due to the new additional presence of deeper focus of infection (abscess inside the super infected EPL).
Pulmonary sequestrations, particularly in children, can also present with features consistent with congestive heart failure, due to left-to-right shunting through the sequestration. More than half of ELS have coexisting congenital malformations, most commonly congenital diaphragmatic hernia and congenital heart disease (total anomalous pulmonary venous return and truncus arteriosus).7, 11-13 In our patient, no coexisting congenital anomaly was found.
Feared complications of pulmonary sequestrations include but not limited to lung abscess, fatal hemoptysis, and torsion necrosis.7, 10 Although extremely few, there are reports of malignancy arising with in both ILS and ELS. One of the hypothesis regarding the risk of malignant transformation in pulmonary sequestrations states that ectopic islets of muscle cells are frequently present inside pulmonary sequestrations and these islets of muscles have the tendency to transform into malignant sarcomas.13, 14
The commonly observed gross appearance of pulmonary sequestrations is a compact lung parenchymal tissue with or without areas of cystic changes. Microscopically, findings are relatively unspecific, and comprise a combination of chronic inflammation and fibrosis. The histopathological differential diagnosis of pulmonary sequestration includes type 1 CPAM, type 2 CPAM, and intrapulmonary bronchogenic cyst. Occasionally, bronchiole-like proliferations could be observed in ELS, which can be mistaken for type 2 CPAM, however, the last one has no systemic feeding vessel, and displays connection with tracheobronchial tree.15, 16
Diagnosis of pulmonary sequestration can be made at different stages. Ultrasonography plays a crucial role for antenatal diagnosis and the subsequent follow up. Fetal echocardiography can also help in identifying collateral vessels supplying sequestrations.17 Enhanced chest CT/MRI is considered as imaging modality of choice to make the diagnosis during infancy and onwards;18 yet, in some cases, chest CT angiography might be additionally required in order to assert the diagnosis as well as for pre-operative planning. In addition, transthoracic needle biopsy (TTNB) can be done before intervention in order to increase pre-procedure diagnostic accuracy.19 In our patient, TTNB could have been done; however, considering the presence of significant radiological evidences in this patient and the high cost of TTNB in our set up, it was not done.
Approach to a patient with persisting pneumonic features should focus on identifying the possible causes such as inadequate antibiotics treatment, complications, neoplasia, infection by unexpected microorganisms (such as mycobacterium tuberculosis, fungus, atypical bacteria), superinfected congenital pulmonary lesions etc.8, 20 In our case, we considered list of differential diagnosis based on patient's age, clinic-radiologic pattern, and specific risk factors. First, we considered the possibility of super infected congenital cystic pulmonary lesions such as CPAM and bronchogenic cyst, but they were all ruled out due to the fact that the mass had arterial nourishment directly emerging from systemic vessel (descending thoracic aorta) which is typical for pulmonary sequestration. Second, the possibility of neoplasia was entertained in this particular patient. However, due to absence of family history, risk factors, and constitutional symptoms of lung cancer in our patient, given the lack of radiologic evidences to suggest malignancy, and the presence of distinct vascular supply to the mass, we ruled out malignant tumor from our top differential diagnosis. Nonetheless, we did not completely neglect the possibility of malignant transformation as it can occur in the background of cystic congenital lung lesions such as pulmonary sequestration. Third, smear negative pulmonary tuberculosis could have provided plausible explanation for our patient's features, especially considering various factors such as patient's history (failure of respiratory symptoms to respond to community acquired pneumonia treatment), the relatively high TB burden in our context, and chest x-ray finding (persisting presence of pulmonary consolidation after antibiotics treatment). In addition, although all the laboratory tests were negative for tuberculosis (Table 1), bronchoscopy wash was not taken in this patient. That was the major limitation in our tuberculosis work up. The summation of these factors increased our diagnostic dilemma. However, the vascular pattern of the mass seen on CT angiography was against tuberculosis. Moreover, even though superinfection of pulmonary sequestration with mycobacterium tuberculosis can rarely occur,21 our patient's histopathology result showed no evidence for tuberculosis infection. In our experience, pulmonary sequestration can present with clinical features masquerading smear negative pulmonary tuberculosis, which could pose diagnostic predicament especially in high TB burden areas lacking well equipped health setting.
The choice of treatment depends on the presence of symptoms and the circumstance in which the diagnosis is made. Prenatally diagnosed pulmonary sequestrations can be followed conservatively till delivery as most of them resolve spontaneously. Surgical resection is often considered as the most effective way to cure symptomatic sequestrations. Although uncommon they are, it is vital to consider post-surgical complications such as air leak, effusion, and infection, if patients show clinical deterioration after surgery.2
In selected patients, who are unfit for surgery or prefer less invasive procedure, endovascular embolization of feeding artery might be used as an alternative to surgery. Possible complications associated with endovascular embolization include incomplete obliteration of feeding vessel leading to reemergence of sequestration, superinfection of dead sequestration after complete occlusion of feeding vessel, and distal migration of dislodged embolization material obliterating non intended vessels. Trans-arterial embolization can be achieved using metallic coils or Amplatzer plugs. Coiling (coil embolization) is the most preferred embolization method according to previous reports, and has been found to yield resolution of pulmonary sequestration with-in a couple of months after the procedure. Nonetheless, there are no follow up studies regarding long-term outcome of coil embolization. On the other hand, endovascular embolization using Amplatzer plugs is recommended particularly for large and short vessels with high flow rate where the risk of coil migration is high.1, 22, 23
The appropriate management of asymptomatic pulmonary sequestrations remains to be a subject of debate. However, most scholars recommend resection of these lesions, given their tendency for superinfection, torsion, and malignant transformation.18
6 CONCLUSION
Pulmonary sequestration is a rare type of congenital pulmonary malformation. The authors suggest that the possibility of pulmonary sequestration should be kept in mind even in young adults having recurrent pneumonitis. Pulmonary sequestration can also mimic smear negative pulmonary tuberculosis, posing diagnostic conundrum. Hence, clinicians with sub-optimal access for CT/MRI angiography need to have high index of suspicion to avoid misdiagnosis and unnecessary anti-tb treatment. Lastly, early surgical resection treats symptoms and prevents the occurrence of serious complications.
AUTHOR CONTRIBUTIONS
Meiraf Bayouh Alemu: Conceptualization. Zenebe Daniel Getachew: Writing- original draft, Writing- review and editing. Adugnaw Atnafu Atalay: Investigation, Validation. Zeru Seyoum Wondimagegn: Investigation. Atsede Birhanu Worku: Project administration. Hiwot Mehari Beyene: Conceptualization. Desalew Gedamu Tareke: Software.
ACKNOWLEDGMENTS
We thank Dr Berhanu G Melka for his input and perspective on this case report.
CONFLICT OF INTEREST STATEMENT
None.
CONSENT
Written informed consent was received from the patient for publication.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated.




