Oral erythema multiforme attributed to herpes simplex virus: A less recognized variant
Abstract
Key Clinical Message
Oral Erythema Multiforme (EM) is considered rare and less described variant in the world of EM. Examination of oral cavity lesions poses various diagnostic challenges and thus a thorough examination with history can help to reach a diagnosis. Due to possibility of subsequent severe attacks of EM, it has to be identified early in the course for better outcome of the patient.
Erythema multiforme is an intriguing clinical entity characterized by acute, self-limiting mucocutaneous hypersensitivity reactions. It can occur due to various etiological factors including infections, medications, autoimmune diseases, and malignancies; herpes simplex being the most common infection accounting for almost 90 percent of the overall cases. Here, we report a case of EM in an 8-year-old female child. The patient presented with an acute onset of crusting ulcers in the lips and oral cavity along with sparse cutaneous lesions around the lips. Based upon the patient's detailed history of prodromal symptoms, disease course and progression, careful clinical examination of wound and serology test, herpes simplex virus-associated erythema multiforme (HAEM) was diagnosed ruling out other mucocutaneous diseases, such as pemphigus, paraneoplastic pemphigus, mucous membrane pemphigoid, and lichen planus. The patient was admitted and treated for HAEM. To conclude, a detail clinical history and thorough clinical examination suggested the diagnosis of EM followed by serology tests to confirm the HSV1 association where proper medicament with supportive care led to an uneventful management.
1 INTRODUCTION
Erythema Multiforme (EM) is an acute inflammatory, self-healing, hypersensitive mucocutaneous disorder characterized by skin eruptions with or without the involvement of other mucous membranes inclusive of oral mucosa.1 It can be induced by certain drug intake, infections, autoimmune diseases, and malignancies. Antibacterial drugs like sulfonamides, penicillins, cephalosporins, quinolones, anticonvulsants, analgesics, nonsteroidal anti-inflammatory drugs, and antifungals are the drugs usually associated with EM, whereas viruses like herpes simplex virus (HSV), Epstein–Barr virus, Cytomegalovirus; Varicella-zoster virus, Hepatitis virus, bacterial pathogens like mycoplasma pneumoniae, mycobacterium, streptococci, fungal agents, and parasites are the common infectious agents associated with EM.2-6 Out of all these, HSV infection is considered as one of the most common predisposing factors of EM and has been identified in up to 70% of the EM diagnosed.7-9
Clinically, EM can be classified as major and minor forms based on the nature and distribution of skin lesions and the degree of mucosal involvement. While EM minor presents with typical target lesions with minimal to no mucosal involvement, EM major shows typical as well as papular atypical target lesions along with occasional bullous lesions in the skin with severe involvement of the mucosal surfaces.10 However, there can also be a subset of EM that affects oral mucosa alone without involvement of the skin and other mucosa.1 Historically, severe forms of EM were described as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis [TEN (Lyell disease)]; however, recent data disproves this suggesting EM to be etio-pathogenetically distinct from those conditions (Table 1).11 When HSV infection is implicated, the diagnosis is bound to be herpes-associated erythema multiforme (HAEM). In these cases, recurrent episodes of EM are usually related to HSV infection.5
| Sub-types of erythema multiforme | Clinical features |
|---|---|
| Erythema multiforme minor |
Typical target lesions, raised atypical target lesions, minimal mucus membrane involvement and, when present, at only one site (most commonly the mouth) Oral lesions; mild to severe erythema, erosions and ulcers. Occasionally may affect only the oral mucosa <10% of the body surface area is affected |
| Erythema multiforme major |
Cutaneous lesions and at least two mucosal sites (typically oral mucosa) are affected. <10% of the body surface area is involved. Symmetrically distributed typical target lesions or atypical raised lesions or both. Oral lesions usually widespread and severe |
| Stevens-Johnson syndrome |
Main difference from erythema multiforme major is based on the typology and location of lesions and the presence of systemic symptoms < 10% of the body surface area is involved Primarily atypical flat target lesions and macules rather than classic target lesions. Generally widespread rather than involving only the acral areas. Multiple mucosal sites involved, with scarring of the mucosal lesions. Prodromal flu-like systemic symptoms are also common. |
| Overlapping Stevens-Johnson syndrome and toxic epidermal necrolysis |
No typical targets lesions, flat atypical targets are present Up to 10%–30% of the body surface area is affected Prodromal flu-like systemic symptoms are common |
| Toxic epidermal necrolysis | When spots are present, it is characterized by epidermal detachment of >30% of the body surface and widespread purpuric macules or flat atypical targets. In the absence of spots, it is characterized by epidermal detachment >10% of the body surface, large epidermal sheets and no macules or target lesions |
EM mostly affects the age groups between 20 and 40 years, with 20% occurrence in children.12 Prodromal signs like fever, malaise, headache, sore throat, rhinorrhea, and cough can be noticed 1 week before the onset of surface erythema which are considered as triggering factors for EM.1 The oral lesions in EM range from mild erythema and erosion to large painful ulcers with an irregular outline and a strong inflammatory halo. Extensive lip involvement with inflammation, ulceration, crusting, and bleeding is typically common of this disease.1
Management of EM depends upon its severity. A mild form of this disease is usually managed using topical analgesics and local wound care, whereas severe cases are controlled with intravenous support using antibiotics, antivirals, and steroids.
2 CASE REPORT
2.1 Case history/examination
An 8-year-old female child presented to the pediatric emergency at B. P. Koirala Institute of Health Sciences (BPKIHS) with a history of wounds on upper and lower lips for 10 days, redness and swelling of gums for 7 days, and inability to eat since last 5 days.
The history of presenting illness revealed the child to have fever 2 weeks back which then proceeded with a small wound on the lips progressing to the entire upper and lower lips. Examination showed extensive involvement of the vermillion borders, and bilateral commissural areas with the perioral areas being involved sparsely. Oral lesions were associated with pain which was severe and mostly aggravated during eating. The patient's past medical history in association with the lesion was not significant along with family and drug history being noncontributory. The patient was well oriented to the time, place and person and the vitals were stable.
Extra oral examination showed sparse ruptured vesicles around the upper lips and chin areas with crusted margins. (Figures 1 and 2) Both right and left submandibular, submental lymph nodes were tender to palpate with the consistency felt as soft to firm.
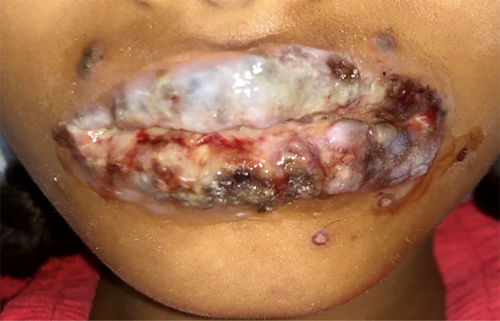
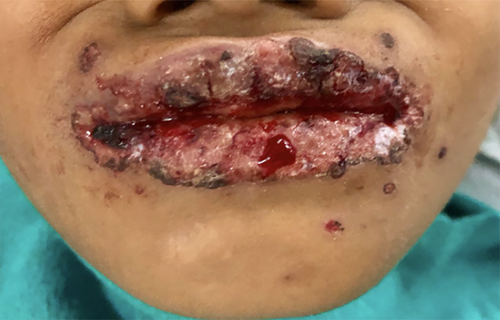
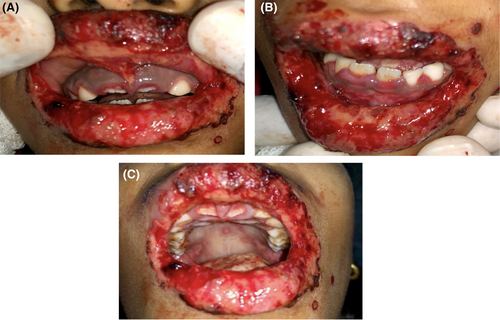
Intraoral examination proceeded following local debridement under topical local anesthesia, which revealed an irregular erythematous area with crustations present on both the upper and lower lips, bilateral commissural areas, and labial mucosa. There was erythematous and swollen gingiva present around the teeth region of 11, 21, 52, and 62. (Figure 3A,B) Multiple ulcerations with irregular margins were present on the hard and soft palate as well with an erythematous halo. The largest of the lesions were noted distal to the teeth 16 and 26 and measured 2 × 1 cm2 approximately (Figure 3C).
3 METHODS (DIFFERENTIAL DIAGNOSIS, INVESTIGATION, AND TREATMENT)
Laboratory investigations revealed increased monocyte level (12%) in complete blood count with decreased sodium level (131 mmol/L). Similarly, blood culture was noncontributory while the erythrocyte sedimentation rate (ESR) was normal with negative serology tested for HBsAg, HCV, and HIV.
Treatment then began with Inj. Ceftriaxone® (750 mg-twice daily), Inj. Acyclovir® (400 mg thrice daily), Inj. Aciloc® (25 mg twice daily), Quadrajel® (thrice daily) and Betadine gargle (four times a day). Wound healing was not satisfactory until the fourth day. So, after the fourth day, Tab. Betnesol® (0.5 mg-crush, swiss, and spit-twice daily−7 days), Gel. Oroheal® (four times a day-2 weeks), Oint. Betasept® (0.5 mg—5 days), Mouthwash Hexidine® (0.2%–5 mL—5 days) and Gel. lidocop® (four times a day-2 weeks) was added to the above-mentioned regimen leading to an uneventful healing. (Figures 4A–C and 5A–C).
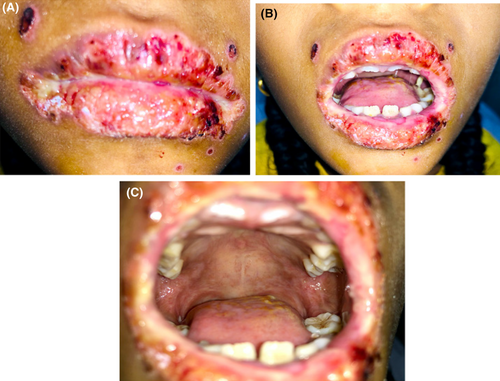
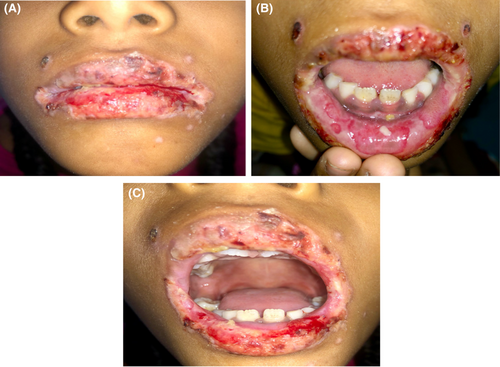
The patient was discharged after 2 weeks of the hospital stay. (Figure 6A–C) Patient serology was tested for the serum antibody against HSV 1 serum IgM and IgG which showed a two-fold increase in IgM level, and more than a 50-fold increase in serum IgG level. Based on the clinical course, patient history, clinical examination, and laboratory findings, the case was diagnosed as Herpes Associated Erythema Multiforme (HAEM).
4 CONCLUSION AND RESULT (OUTCOME AND FOLLOW UP)
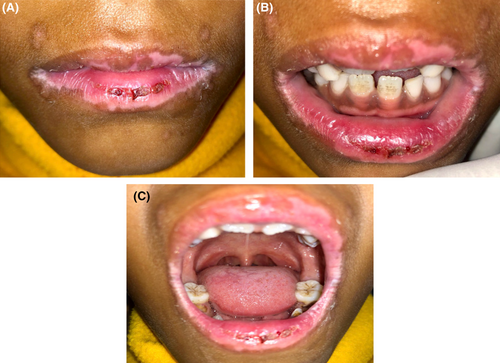
Detailed history, thorough clinical examination along with laboratory tests in combinations are the keystones for the diagnosis of EM. In the present case, parental and patient detail history along with thorough clinical examination established the clinical diagnosis of EM followed by serological tests, which confirmed HSV1 association leading to the diagnosis of HAEM. A palliative cure with proper medicament led to the uneventful healing without reoccurrence till 6 months follow-up. (Figure 7A–C) However, regular follow-up for the possibility of reoccurrence along with the need for long-term low-dose antiviral therapy should been considered.
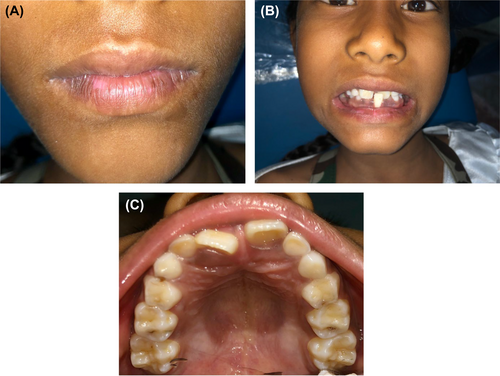
5 DISCUSSION
HSV infections are the most commonly identified cause of EM that includes both HSV types 1 and 2.7 HSV DNA has been detected in 60% of patients clinically diagnosed with recurrent HAEM and in 50% of patients with recurrent idiopathic EM using polymerase chain reaction (PCR) of skin biopsy specimens.13 Typical presentation of an EM (minor or major) lesion begins 10–14 days following the clinical manifestations of an HSV infection, the lip being the most common site of infection.4 The serology for HSV1 was positive in this patient thus, confirming that the EM was associated with an HSV1 infection.
Several studies in the past have demonstrated the pathogenesis of HAEM consistent with a delayed hypersensitivity reaction.14, 15 Peripherally circulating antigen-presenting cells (Langerhans cell precursors with CD34+ cells) transport HSV DNA to the keratinocytes leading to the activation of HSV-specific CD4+ TH1 cells. The activated CD4+ cells release interferon-γ (IFN-γ) which then initiates the inflammatory cascade in response to the viral antigen. This activation of the inflammatory cascade then led to the immune-mediated epidermal damage.16 The presence of HSV DNA in any lesions and tissues can be detected through PCR. Another test such as reverse transcriptase PCR or immunohistochemistry using antibodies to specific viral genes can also be used for the detection of HSV gene. The presence of HSV-1 and HSV-2 genes in serology along with detection of IgM and IgG antibodies specific against HSV is a confirmatory test result to diagnose EM associated with HSV.6 In the present case, IgM and IgG antibodies specific against HSV was detected.
The diagnosis of HAEM is clinical and is easier when the patient develops target lesions with a preceding or coexisting HSV infection. The finding of typical skin or oral lesions (or both) in a patient with suspected HAEM supports the clinical diagnosis.
The oral manifestations of EM range from mild tender superficial erythema to painful hemorrhagic bullae and erosions.1 oral lesions usually start with swelling, redness, and macules on the lips and buccal mucosa which ruptures and lead to pseudomembrane formation along with diagnostically distinctive bloody excrustation formation in lips. Intact vesicles are very hard to observe clinically as they rapidly rupture to form ulcers. Target lesions can be seen on the lip but rarely on the intraoral mucosa.17 The oral mucosa is the most commonly involved mucosal surface in EM however any mucosal site can be affected.18
There is no reliable laboratory based mean of definitively diagnosing EM. Diagnosis usually done by excluding other similar diseases by careful review of the clinical history and detailed clinical examination. The diffuse and widespread oral ulceration of EM can be difficult to differentiate from other vesiculobullous disorders such as pemphigus or pemphigoid. EM should also be differentiated from viral stomatitis and toxic epidermal necrolysis.18 Clinical features of EM are the acute onset (or recurrent nature), oral lesions typically located on the lip, and anteriorly in the mouth with formation of excrustations along with formation of target lesions. In the present case, the history of lesion development and the involvement pattern of the intraoral sites along with detection of the serum antibody against HSV led to the diagnosis of HAEM.
Treatment strategy for HAEM depends upon the severity of the lesion. In milder form, topical analgesics for pain control, proper wound dressing along with soft diet generally leads to uneventful wound healing in two to six weeks time. However, severe cases might require intensive management with intravenous fluids and drugs.7 Systemic corticosteroids have been used successfully in some patients, but evidence to support their use for EM is limited.5 Reports show that drugs such as Thalidomide, Dapsone, Levamisole, and Azathioprine can be used if systemic corticosteroids and acyclovir fail to treat the case.19-21 In the present case, management was done with intravenous antibiotics, and antiviral drugs along with topical corticosteroids.
HAEM resolves spontaneously in 10–20 days. However, there is a high chance of reoccurrence. Reports show that around 20%–25% of EM patients may experience multiple episodes of reoccurrence ranging from minimum two to two dozen episodes a year.13 The symptoms can range from mild to severe involvement of the oral mucosa and mucocutaneous area along with skin respectively.12 It is always important to follow symptomatic management of the patient that is, pain control, hydration and prevention of secondary infections along with an appropriate referral to the specialist to prevent and manage if systemic complications like extraoral EM, SJS, TEN emerge.2, 4, 15 HAEM is often effectively managed with acyclovir (200 mg, five times a day for 5 days), but only if the therapeutic scheme can be started in the first few days.2, 9, 22 For recurrent cases, a continuous low dose of oral acyclovir is necessary.4, 7, 14 Oral acyclovir is effective in preventing recurrent HAEM with protocols including 200–800 mg/day for two to six weeks. If acyclovir treatment fails, valacyclovir is the next recommended drug (500 mg twice a day).4, 14
ACKNOWLEDGMENTS
The authors would like to acknowledge the Department of Oral Medicine and Radiology, Department of Pediatrics and Adolescent Medicine and Department of Dermatology for providing valuable inputs and help during the entire treatment procedure.
AUTHOR CONTRIBUTIONS
Santosh Dharel performed the clinical procedure under the guidance and supervision of Professor Dr. Bandana Koirala and Associated Professor Dr. Mamta Dali. Santosh Dharel wrote the manuscript. All authors contributed to the editorial changes along with the reading, editing, and approval of the manuscript.
FUNDING INFORMATION
This research received no external funding.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
CONSENT
Written informed consent was obtained from the patient's mother for publication of this case report. A copy of written consent is available for review by the editor- in- chief of this journal. Consent from mother was obtained as patient was below legal age to consent.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




