Diagnostic challenge of an untypical cutaneous drug reaction from modafinil
Key Clinical Message
Modafinil used for narcolepsy caused an atypical adverse reaction, as itchy acneiform lesions, subcutaneous nodules and superficial skin wounds after 2 years of use. After extensive examinations for 5 years, modafinil was discontinued, and the healing process began in 3 weeks with completion in 1.5 years.
1 INTRODUCTION
Modafinil is a non-amphetamine CNS-stimulant promoting wakefulness licensed widely in Europe with varying indications. Sleepiness associated with narcolepsy is the only indication approved in all EU countries, where it is used.1
Modafinil was first authorized in France in June 1992. The mechanism of action is not entirely understood, but the most consistent findings of the various studies performed are the inhibitory effects on the dopamine transporters.2
The data from clinical trials have raised concerns related to serious psychiatric and dermatologic disorders. The product information for modafinil has been updated across Europe to include strengthened warnings.2
However, serious skin adverse reactions are very rare. In clinical modafinil studies, there are reportedly zero cases in 4264 patients. In 1585 pediatric patients aged 17 years of less, there were 13 cases of skin adverse reactions, including serious ones.3
Modafinil has been associated with dermatological adverse effects, like urticaria and angioedema and anaphylaxis, acne, itch, skin rash with eosinophilia (DRESS), exfoliative dermatitis, and hyperhidrosis that have been reported in addition to Stevens–Johnson syndrome, toxic epidermal necrolysis and erythema multiforme.2-4
A clinical study involving 122 patients under modafinil treatment found that 25 of them had to discontinue the medication. Interestingly, one patient experienced sleepiness, an increase in hallucinations, and acne as adverse effects. However, the age of the patient is not known.5
Acneiform drug reactions are described in the Dermatology Textbook6 from a variety of drugs presenting mostly in face, chest, and back. These drugs include corticosteroids, hydantoin, and lithium as the most common causes. The less common mentioned drugs include oral contraceptives, androgens, halides, isoniazid, ethanamide, rifampicin, tetracyclines, disulfiram, barbiturates, cyclosporine, vitamins B2, B6, B12, and psoralen UVA-treatment.
Here we describe a challenging evaluation of adverse reaction to modafinil presenting with itchy, partially acneiform dermatitis and itch-related scratching artifacts and pruritic subcutaneous nodules, starting after 2 years of regular use.
2 CASE HISTORY, EXAMINATION
A 34-year-old female patient had a history of hypersomnia, somnambulism, and daytime drowsiness since childhood. She had also undergone numerous surgical operations, including a tonsillectomy, left ovarian removal due to mucinous adenoma, acute volvulus surgery because of intestinal malrotation, and bilateral median nerve release.
The diagnosis of narcolepsy type 2 without cataplexy was established after adequate neurophysiologic examinations in December 2013, when the patient was 25 years old. She had been living in Finland, Sweden, the Middle and Far East and Central Europe before and after the diagnosis. Modafinil (400 mg in the morning) was started in February 2014 with positive response to both day- and nighttime symptoms. With the support of modafinil the patient was able to commence and complete her academic studies and carry on an active life. Her clinical condition was followed up by a neurologist on a regular basis. Citalopram has been used as medication.
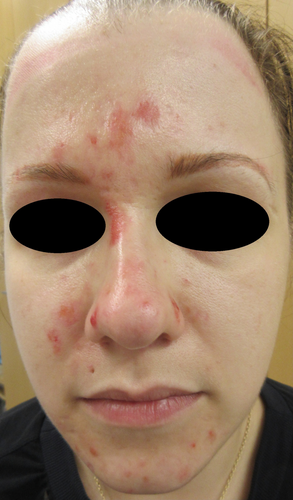
In February 2016, papular acneiform dermatitis lesions were noticed first in the facial area (Figure 1). Subsequently in weeks, lesions were widely seen also all over the body and extremities. Staphylococcus aureus was cultured from several dermatitis lesions, and there were panniculitis-like lumps in lower limbs. The patient exhibited generalized malaise and experienced persistent low-grade fever. Notably, the patient had no history of atopy.

3 DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENTS
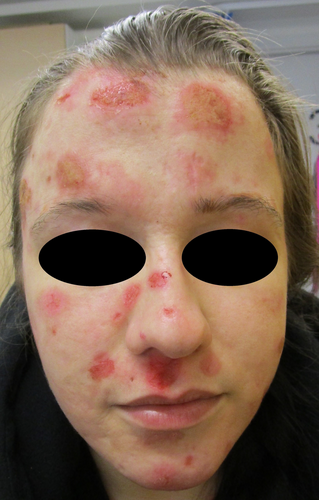
A wide variety of topical medications were used in the treatment, including antimicrobial wet compresses, 0.05% clobetasol propionate cream, 2% fucidic acid cream, hydrocortisone 1% cream and pimecrolimus 0.1% ointment. In addition, oral prednisolone up to 10 mg/day, dapsone 50 mg/day, methotrexate up to 15 mg/week with 5 mg of folic acid were tested. None of the topical or oral treatments resulted in any remarkable relief (Figure 2).
In 2019, a rheumatologist was consulted because of multiple joint pain and suspicion of panniculitis without a specific diagnosis for connective tissue diseases. Nonetheless, low-dose prednisolone (7.5–10 mg/day) alleviated the pain symptoms. Concurrently, left wrist de Quervain tenosynovitis was parallelly treated with oral prednisolone. A broad panel of serologic tests related to systemic vasculitis and autoimmunity were performed, and an autoimmune thyroiditis with marginal hypothyroidism was diagnosed based on temporarily elevated thyroid autoantibodies and low T4-V level. However, an oral thyroxin treatment was not started. There were no other specific findings in these numerous serum analyses, shown as supplementary material (Table 1A,B). In addition, numerous skin biopsies for histopathology were taken from the forehead skin lesion by knife and divided to light microscopic and immunofluorescence studies, the latter was repeated by a 3 mm punch biopsy. Also 3 mm punch biopsies were taken from left lateral cheek, from the right anterior and left posterior aspect of thighs and left thumb. Also, a deep biopsy from a subcutaneous nodule in left thigh was taken. These studies revealed only unspecific dermatitis and secondary vasculitis with no signs of primary vasculitis. A genetic HLA-analysis of known variants associated with narcolepsy produced no diagnostic findings. In addition, an Oto-Rhino-Laryngology clinic was consulted with biopsy from left nose lower crust with result of mild inflammation.
During the follow-up, the clinical condition worsened dramatically. Therefore, the patient was evaluated in a multi-professional rare disease panel consisting of a dermatologist, rheumatologist, geneticist, gynecologist, pathologist, psychiatrist, psychologist, and a specialist in internal medicine. The skin lesions were itchy, and the patient could not refrain from scratching them despite of cetirizine was taken 4 times a day; serum IgE was very low 1.0 kU/L (ref. range 0–100 kU/L).
Therefore, many of the consulted domestic and foreign specialists suspected a self-inflicted artifact disease with a psychiatric basis. The patient did not exhibit psychiatric disturbances linked to modafinil use. A throughout psychiatrist evaluation did not show any self-inflicted psychiatric basis for skin dermatitis.
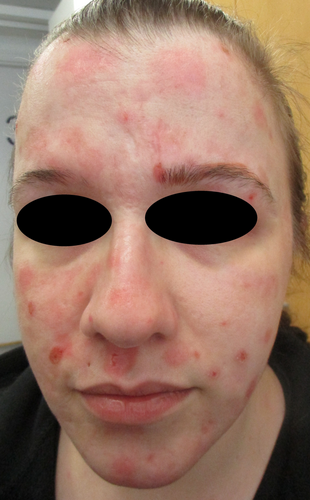
In the multi-professional meeting, a possibility of adverse drug reaction was considered, too. Thus, modafinil was decided to be discontinued by the dermatologist after 7 years of regular use and consultation of a neurologist in February 2021 (Figure 3). Consequently, skin lesions started to heal within 3 weeks (Figure 4), and after 1 year there were only some scars left. Also, the subcutaneous nodules disappeared within a few months.
4 OUTCOME AND FOLLOW-UP
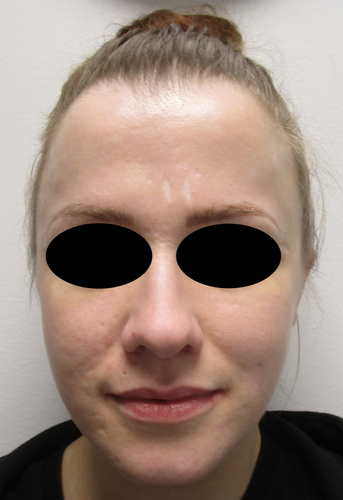
In April 2021, pitolisant, a histamine H3 receptor antagonist and inverse agonist, was started with a dose of 18 mg once a day. At the neurologist follow-up visits of narcolepsy, the patient has been satisfied at pitolisant's effect without any remarkable adverse effects. At the dermatologist follow-up visit in June 2023, the patient's skin remained healed (Figure 5). By phone conversation in May 2024, the patient informed that her skin condition has remained excellent. Also, the most marked scars after ultra-pulsed CO2-laser treatments have gone to almost invisible.
5 DISCUSSION
Diagnosis of delayed drug reaction to modafinil was very challenging presenting with itchy, partially acneiform dermatitis and itch-related scratching artifacts and pruritic subcutaneous nodules, starting after 2 years of modafinil use. These kinds of symptoms may also be a clinical sign of psychogenic artifact. However, this was considered not to be the case in our patient as assessed by the multi-professional team.
Phototoxic reactions are not likely, since in addition to facial area, similar lesions were noticed also in the trunk, upper and lower extremities, which areas were covered by clothes. Also, the patient did not take sun baths, undergo light therapies, or use light-sensitizing medications or topical products. Citalopram has been used before and after discontinuation of modafinil.
In the literature, most adverse effects have been diagnosed after a relatively short period of use, that is, within less than 5 weeks,5, 6 but in some cases within 3 months.6 In this case, modafinil was used over 2 years before the skin lesions appeared, and it was discontinued after 5 years of use. Thus, we suspect very strongly based on clinical finding that the cause was modafinil to promote skin acneiform dermatitis that—after discontinuation modafinil—started to heal rather rapidly, and itch disappeared in a few days. Also, the subcutaneous nodules disappeared within a few months.
The patient has been satisfied with pitolisant now for over 3 years. After suffering from severely itchy dermatitis for 5 years and hampering markedly her personal and work life, she did not consider relevant to make a re-challenge with modafinil to confirm the diagnosis. In addition, the patient moved to another city/country. Thus, epicutaneous patch tests and/or lymphocyte stimulation test were not possible to perform, though these would have been a nice additional investigation in this case. However, it is just speculative, whether these studies would still have produced any positive result.
It is noteworthy that there were no such typical findings for adverse drug effects formerly reported. Nevertheless, the rapid healing of skin lesions started within 3 weeks after discontinuation of modafinil supporting our conclusion of a modafinil adverse effect, which is in accordance with the elimination half time of about 15 h,3 thus eliminated within 6–7 days.
6 CLINICAL MESSAGES
- During modafinil use, a prolonged acneiform dermatitis with subcutaneous nodules and superficial wounds were detected, not reported earlier.
- Superficial wounds with secondary Staphylococcal colonization are likely partially linked to antihistamine-and topical antimicrobial steroid-resistant itch-related scratching.
- Modafinil is linked in our patient to an adverse skin reaction even after 2 years of prolonged use when adverse events are reported within 3 months in the literature.
- After discontinuing modafinil, the healing started in a few weeks.
- Psychiatric self-inflicted cause was excluded by an extensive psychiatrist evaluation.
AUTHOR CONTRIBUTIONS
Rauno J. Harvima: Formal analysis; investigation; methodology; project administration; supervision; validation; visualization; writing – original draft; writing – review and editing. Tarja Heiskanen-Kosma: Formal analysis; investigation; methodology; validation; writing – original draft; writing – review and editing. Miika Arvonen: Formal analysis; investigation; methodology; writing – original draft; writing – review and editing. Ilkka T. Harvima: Formal analysis; investigation; methodology; writing – original draft; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
ETHICAL APPROVAL
The patient has given her written consent for this case report. She has proof-read the final submitted manuscript in which her comments have been exactly implemented.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




