Combined sepsis- and renal dysfunction-induced hypoglycaemia in an older patient taking cibenzoline
Abstract
Key Clinical Message
An 86-year-old geriatric patient with sepsis presented cibenzoline-induced hypoglycaemia, although within the boundary range of cibenzoline blood concentration.
An 86-year-old geriatric patient taking cibenzoline for ametropic hypertrophic cardiac tendinopathy was admitted to our hospital for the treatment of sepsis. Upon admission, blood cibenzoline levels of 400.1 ng/mL were observed. Antibiotic therapy was initiated and cibenzoline was discontinued. On Day 16, cibenzoline was administered orally at a reduced dose of 50 mg/day. Several days after restarting cibenzoline, the patient developed hypoglycaemia (64 mg/dL), prompting the administration of 20% glucose. The present case demonstrates a rational timeline for cibenzoline administration, considering the patient's renal dysfunction and sepsis. Clinicians should exercise caution when managing older patients with severe infections who are receiving cibenzoline, and should consider the possibility of blood glucose fluctuations regardless of cibenzoline blood levels. Further research is warranted to better understand and address the potential side effects of cibenzoline administration in geriatric populations.
1 INTRODUCTION
Cibenzoline, a class Ia antiarrhythmic drug, is known to be excreted in the urine, with approximately 55%–62% of the administered amount unchanged.1, 2 Owing to its non-linear pharmacokinetic pattern, cibenzoline is classified as a drug for therapeutic drug monitoring (TDM), the blood level of which is recommended to be under 250 at trough −800 ng/mL at peak to avoid toxicity.3, 4 Hypoglycaemia, a common adverse event caused by cibenzoline, poses high risk in patients with high level of cibenzoline of around 700–3000 ng/mL, and reported to avoid the risk for adverse effects for under 400 ng/mL at trough concentration as reported in previous case reports and guideline.3, 4 Although the risk factors were reported for impaired renal function or older individuals,5-10 the information of cibenzoline toxicity undergo boundary range to avoidable cibenzoline toxicity is lacking. Herein, we present the case of a patient who experienced hypoglycaemia within the boundary range to toxicity of cibenzoline blood concentrations, triggered by sepsis-induced renal dysfunction.
2 CASE HISTORY
An 86-year-old woman (weight: 44.6 kg, height: 150.0 cm) with a medical history of hypertension, ametropic hypertrophic cardiac tendinopathy, and Parkinson's disease underwent spinal fusion surgery for a compression fracture of the 12th thoracic vertebra at Showa University Koto Toyosu Hospital in September 2021. Four weeks after surgery, the patient was transferred to a hospital specializing in rehabilitation. After 2 weeks, she presented with a mildly decreased level of consciousness (Glasgow Coma Scale: E4V4M5) and was urgently transferred to our hospital owing to sepsis.
Upon admission, her laboratory data showed a creatinine clearance of 33.4 mL/min with a body temperature of 34.7°C (blood pressure: 124/74 mmHg, heart rate: 61 beats/min) on Day 1. On the same day, she experienced hypotension (69/41 mmHg) and a further decline in her level of consciousness in the emergency department.
3 METHODS
We first administered tazobactam and piperacillin (TAZ/PIPC: 2.25 g), four times a day as empiric therapy on Day 1. During this period, the patient continued to receive oral medications, including celecoxib (200 mg/day), famotidine (10 mg/day), cibenzoline (100 mg/day), levodopa (300 mg/day), ramelteon (8 mg/day), magnesium oxide (990 mg/day), and sennoside (12 mg/day). Olmesartan (10 mg/day), amlodipine (2.5 mg/day), and carvedilol (10 mg/day) were discontinued due to hypotension.
On Day 2, her creatinine clearance further decreased to 26.5 mL/min, prompting us to request the outsourcing of the cibenzoline blood concentration measurement, considering her renal impairment. On Day 2, we added 260 mg of daptomycin every 48 h to her medication list for Gram-positive cocci, after receiving her blood culture results. On Day 3, owing to a decrease in casual blood glucose levels to 56 mg/dL, 20 mL of 20% glucose was administered. Additionally, her fasting blood glucose level was low (62 mg/dL) on the same day. Subsequently, her blood glucose levels were closely monitored and glucose was administered as needed to maintain appropriate levels.
On Day 6, cibenzoline was discontinued because the outsourcing results indicated a trough blood concentration of 400.1 ng/mL. Additionally, we discontinued TAZ/PIPC treatment upon detection of methicillin-resistant Staphylococcus aureus (MRSA) in the bloodstream, based on the results of the blood culture performed on Day 1. Throughout this period, her blood glucose levels ranged from 86 to 242 mg/dL and insulin was administered as necessary to regulate her blood sugar levels. By Day 16, both her blood glucose and serum creatinine levels improved, prompting us to restart cibenzoline at a reduced dose of 50 mg/day, considering her hypertrophic cardiac tendinopathy. On Day 36, the dosage of cibenzoline was increased to 100 mg/day after confirming a low blood concentration of cibenzoline (56.4 ng/mL) from an outsourced measurement taken on Day 26. She had a urinary tract infection caused by β-lactamase Klebsiella pneumoniae, as tested from urine sample on Day 40. Therefore, we changed her medication from daptomycin to meropenem (0.5 g, three times a day). From clinical observations, we did not implement blood culturing at the time. On Day 43, her creatinine clearance and fasting blood glucose levels deteriorated again to 26.6 mL/min and 64 mg/dL, respectively. However, cibenzoline blood concentration on Day 44 was within the therapeutic range (203.7 ng/mL). On Day 52, the patient's creatinine clearance improved from 26.5 mL/min (Day 2) to 47 mL/min (Day 52). We discussed with the cardiologist and considered the benefit and risk of re-starting cibenzoline administration because of her difficulty in controlling blood sugar levels, independently against cibenzoline blood concentration. Finally, we decided to discontinue further administration of cibenzoline (Figure 1) (Table 1).
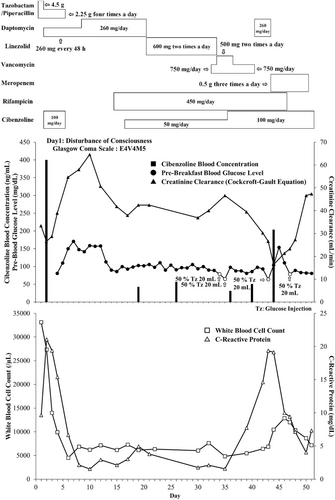
4 CONCLUSION AND RESULTS
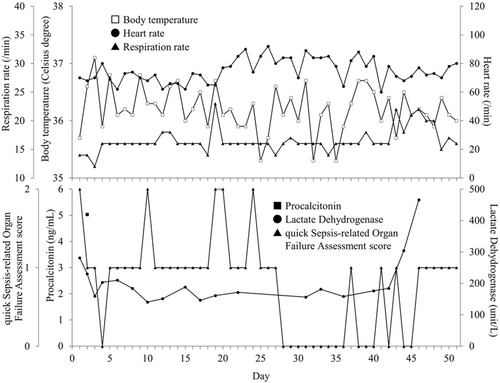
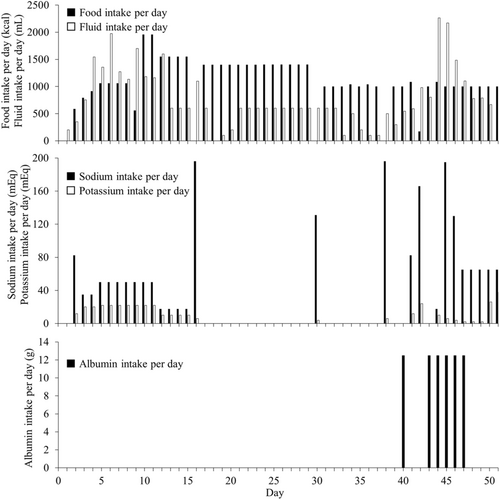
Following the discontinuation of cibenzoline, no further episodes of low blood glucose were observed. Ultimately, the patient's sepsis improved (Figure 2). Moreover, no problem in the intake of food, fluids, albumin, and electrolytes were observed (Figure 3). Finally, she was transferred to a convalescent rehabilitation hospital on Day 110.
| Day 1 | Tazobactam/Piperacillin | 4.5 g/day | Day 21–33 | Linezolid | 600 mg two times a day |
| Day 2–5 | Tazobactam/Piperacillin | 2.25 g four times a day | Day 22–35 | Memantine | 10 mg/day |
| Cibenzoline | 100 mg/day | Day 33 | Vancomycin | 750 mg/day | |
| Day 2–8 | Daptomycin | 260 mg every 48 h | Day 34–36 | Vancomycin | 500 mg two times a day |
| Day 2–46 | Levodopa | 300 mg/day | Day 36 | Memantine | 15 mg/day |
| Celecoxib | 200 mg/day | Day 36–51 | Cibenzoline | 100 mg/day | |
| Famotidine | 10 mg/day | Day 37–40 | Vancomycin | 750 mg/day | |
| Magnesium oxide | 660 mg/day | Day 41–43 | Daptomycin | 260 mg/day | |
| Day 3–46 | Sennoside | 12 mg/day | Day 44–46 | Tolvaptan | 3.75 mg/day |
| Ramelteon | 8 mg/day | Day 44–50 | Meropenem | 0.5 g three times a day | |
| Day 8–46 | Furosemide | 20 mg/day | Day 46–49 | Furosemide | 10 mg/day |
| Day 9–20 | Daptomycin | 260 mg/day | Day 47 | Tolvaptan | 7.5 mg/day |
| Day 15–46 | Rifampicin | 450 mg/day | Day49 | Nifedipine | 20 mg/day |
| Day 17–35 | Cibenzoline | 50 mg/day | Day50 | Furosemide | 40 mg/day |
5 DISCUSSION
In this case report, we observed the occurrence of cibenzoline toxicity leading to hypoglycaemia in a geriatric patient with severe infection during the presented period, despite the cibenzoline blood concentration being within the boundary range between toxic and therapeutic level.
In reality, hypoglycaemia is reported in patients with sepsis, with an incidence of approximately 6%–10%, and is often associated with increased severity and high mortality in cases of severe sepsis.11-14 The mechanism underlying sepsis-induced hypoglycaemia involves a deficiency of the hormones cortisol and adrenaline, enhanced inhibition of gluconeogenesis in the liver, and increased peripheral glucose utilization.12, 15 These factors may have contributed to hypoglycaemia in our patient, given the presence of sepsis. In addition to the infection-based hypothesis, we considered her hypoglycaemia to be potentially affected by her lower renal function. Hypoglycaemia induced by cibenzoline is higher in patients with renal dysfunction.16-18 Cibenzoline-induced hypoglycaemia has been reported to be related to the blood concentration of cibenzoline.3, 19 To regulate cibenzoline between the therapeutic range of 250–800 ng/mL, dose reduction regimens are cited in the package insert, and TDM is recommended because of the drug's non-linear pharmacokinetic pattern.3, 4 In our case, the patient's base creatinine clearance was around 46 mL/min. We administered cibenzoline at a dose of 100 mg/day following the guidelines provided in the package insert. However, despite maintaining blood concentrations of cibenzoline within the therapeutic range, such as 400.1 ng/mL on Day 2 and 203.7 ng/mL on Day 44, the patient experienced recurrent episodes of hypoglycaemia, suggesting a reasonable relationship with cibenzoline administration.
Oda et al. previously reported cases of cibenzoline-induced hypoglycaemia occurring within the therapeutic range.20 Bertrand et al., Takahashi et al. hypothesized that cibenzoline directly stimulates pancreatic beta cells, leading to increased insulin secretion, as observed in animal models. This provides a potential mechanism for cibenzoline-induced hypoglycaemia.21, 22 Unfortunately, we did not implement insulin measurement in this case, but these proposed mechanisms may explain the occurrence of hypoglycaemia in our patient, despite maintaining cibenzoline blood concentrations within the therapeutic range.
As a limitation, we could not obtain the patient's blood samples within the time course after administration of cibenzoline. Cibenzoline exhibits a non-linear pharmacokinetic pattern. In our case, cibenzoline blood concentrations ranged between 56.4 and 400.1 ng/mL when the patient was administered cibenzoline at 50 and 100 mg. From our blood sampling timing, we could not assess if the changes in the blood concentration of cibenzoline were dependent on its non-linear pharmacokinetic pattern or other factors including sepsis. In other words, when administering cibenzoline, clinicians should always be careful about hypoglycaemia, regardless of its blood concentration.
6 CONCLUSION
In conclusion, this case report highlights the importance of recognizing cibenzoline toxicity as a potential cause of hypoglycaemia, even when the blood concentration of the drug is under the toxic range. The concurrent presence of sepsis and renal dysfunction likely contributed to the development of hypoglycaemia in our patient. Clinicians should exercise caution when managing older patients with severe infections who are receiving cibenzoline, as it is necessary to consider the possibility of blood glucose fluctuations regardless of cibenzoline blood levels. Further research and monitoring of cibenzoline use in geriatric populations is warranted to better understand and manage its potential side effects.
AUTHOR CONTRIBUTIONS
Tatsuki Iha: Data curation; writing – original draft. Ayako Watanabe: Conceptualization; data curation; investigation; methodology; writing – original draft. Misa Saeki: Data curation; validation. Ayaka Itoh: Data curation; investigation; validation. Yuka Kashiwabara: Supervision. Akiko Fujiwara: Supervision. Kenji Momo: Conceptualization; methodology; project administration; supervision; writing – original draft.
ACKNOWLEDGMENTS
None.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The Department of Hospital Pharmaceutics, School of Pharmacy, Showa University received funds from Ono for a contract research project according to a collaborative research agreement. As a potential conflict of interest, KM received honorarium fees for presentations from Abbvie, Nippon-Kayaku and Eisai. And received a travel fee from Abbvie to join the conference held by Abbvie. YK received an honorarium fee for the presentation at Daiichi Sankyo and Chugai. The department of Hospital Pharmaceutics received research grants from Nippon-Kayaku, Ono, Shionogi, Bayer, Daiichi Sankyo, Eisai, Mochida, and Taiho. The other authors declare no conflict of interest associated with this manuscript.
ETHICS STATEMENT
None.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
All information in this case is included in this published article.




