Scalp metastasis from atypical meningioma: A case report and literature review
Abstract
Key Clinical Message
Scalp metastasis from atypical meningioma, though rare, underscores the importance of meticulous surgical techniques to prevent tumor cell implantation. Early detection and comprehensive management, including surgery and adjuvant therapy, are crucial for optimal outcomes.
Meningiomas are tumors of the meninges that originate in the arachnoid layer from arachnoid cap cells. Atypical meningiomas, classified as WHO grade 2 tumors, tend to metastasize and recur if not surgically managed properly. Scalp metastasis is a rare occurrence that presents as a subcutaneous elevation. A 33-year-old patient presented with a complaint of a constant, dull pressure headache persisting for the past 12 months, exacerbated by exertion, along with seizures and neuropsychiatric symptoms. The patient had no significant medical history but had undergone surgery 4 years ago for a WHO grade 2 meningioma. The current brain MRI revealed a dural tail sign, along with masses on both the left and right sides of the frontal lobe, extending to involve the skin on the forehead and scalp. The patient underwent surgical resection and adjuvant radiation therapy. At the 12-month follow-up, no neurological deterioration or tumor recurrence was observed. A literature review on scalp metastasis in patients with atypical meningioma was also conducted, including eight articles published up to September 2023. The mechanism of metastasis development appears to be consistent in all eight reported cases, involving the implantation of tumor cells during resection. Therefore, there is a critical need for meticulous intra- and post-operative surgical techniques to prevent such implantation.
1 INTRODUCTION
Meningiomas account for a substantial proportion of benign tumors (53.3%) and primary brain tumors (38.3%) in the United States.1 According to the World Health Organization (WHO) classification, meningiomas are categorized into three grades: benign (grade 1, 89.30%), atypical (grade 2, 5.90%), and anaplastic (grade 3, 4.80%) meningiomas.1 Atypical meningiomas, classified as WHO grade 2, are characterized by their metastatic potential and proclivity for recurrence, presenting significant challenges in their surgical management and treatment.2
Meningioma metastasis primarily occurs through general circulation or iatrogenic distribution.3, 4 Skin metastasis from internal organ cancers accounts for 5%–9% of cases.5 Metastasis from malignant meningiomas is exceedingly rare, further underscoring the rarity of scalp metastasis. One established pathology is iatrogenic metastasis resulting from the resection of atypical meningiomas, emphasizing the importance of meticulous surgical techniques to minimize the risk of metastatic recurrence.6 This study presents a case of scalp metastasis from atypical meningioma and provides a comprehensive literature review on the topic.
2 CASE HISTORY/EXAMINATION
A 33-year-old female patient presented to our tertiary care hospital in 2017 with a complaint of persistent headaches for 12 months. She described the headaches as dull and pressure-like, worsened by coughing, sneezing, and straining. Additionally, she experienced occasional generalized tonic–clonic seizures and exhibited behavioral changes such as depression, memory disturbances, anxiety disorder, and paranoid ideation. The patient had no other significant medical history.
3 METHODS (DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS AND TREATMENT)
Brain magnetic resonance imaging (MRI) revealed a large meningioma located in the left frontal convexity, and histopathology confirmed it as a WHO grade 2 meningioma. The recommended treatment plan involved radiation therapy and chemotherapy, followed by surgical resection of the meningioma. However, the patient only underwent tumor excision at another tertiary care hospital and did not receive radiation or chemotherapy, resulting in tumor recurrence.
Four years later in 2021, the patient returned to our hospital with similar complaints, along with tumor involvement on the left side of the forehead skin and scalp. On physical examination, a fixed, firm, tender lesion measuring approximately 10 × 12 cm on the left side and 7 × 7 cm on the right side was observed (Figure 1A). No surrounding lymph nodes were affected, and there were no additional lesions on other parts of the body. CT scan showed bone erosion (Figure 1B). Brain MRI showed a broad-based intra- and extra-axial lesion involving the frontal lobe, with associated mass effect, scalp erosion, and a “dural tail sign” (Figure 1C).

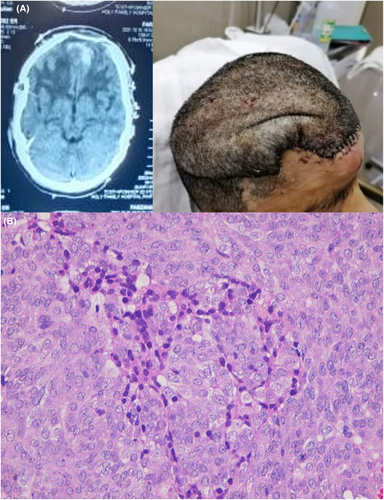
To address the recurrence, the patient underwent tumor excision via a left frontal craniotomy in the elective operating room of the neurosurgery department. During the procedure, the eroded frontal bone was also removed, and frozen sections were examined urgently in the emergency department to ensure a positive margin of tumor resection. After approximately 6 h, a “Simpson grade I” resection of the tumor was achieved. To reconstruct the skull, titanium mesh placement was performed in collaboration with the plastic surgery department, followed by skin grafting. Immediate postoperative brain CT scan showed a complete removal and good cosmetic results (Figure 2A). Histopathological analysis revealed a meningioma with zones of increased cellularity, necrosis, and pleomorphism, consistent with the diagnosis of an atypical meningioma (Figure 2B).
4 CONCLUSION AND RESULTS (OUTCOME AND FOLLOW-UP)
Postoperatively, radiation therapy was administered to the excision site with wide margins. The patient experienced immediate improvement in her symptoms and remained neurologically stable. Follow-up MRI at 12 months showed no signs of tumor recurrence.
5 DISCUSSION
A literature review was conducted to compile all case reports on scalp metastasis resulting from atypical (WHO grade 2) meningioma published up to September 2023. The search strategy included keywords such as “WHO grade 2 meningioma,” “atypical meningioma,” “metastatic meningioma,” “malignant meningioma,” and “scalp metastasis” or “skin metastasis.” Due to the rarity of this condition, all eight studies included in the review were case reports. In all reported cases, patients underwent resection and received radiotherapy, resulting in a 100% survival rate at a mean follow-up of 18 months.
Meningiomas account for approximately 15.60% of all primary CNS tumors treated in Pakistan.7 The vast majority of meningiomas are nonmalignant (about 90%).8 Malignant meningiomas represent roughly 0.4% of all CNS tumors in the United States.9 Atypical meningiomas, classified as WHO grade II, exhibit a characteristic tendency for recurrence.10 The incidence of atypical meningiomas, along with meningiomas in general, is notably higher among female patients both globally11 and in Pakistan.12
Scalp skin metastasis from atypical meningioma is an exceptionally rare clinical phenomenon, with only eight reported cases in the literature, making the presented case the ninth and the second from the same country (Table 1). The plausible mechanism underlying metastasis is the dispersion of meningioma cells during surgical resection. Other factors associated with metastatic meningiomas include reoperations, immunosuppression, radiation therapy, and a protracted course of the surgical incision with a cerebrospinal fluid fistula.13-16 Knowledge of these factors is valuable for neurosurgeons, despite the rarity of scalp metastasis in meningiomas. The median time interval between diagnosis and scalp metastasis presentation was 44.5 months, primarily explaining the iatrogenic seeding of cells. The presence of large subcutaneous lesions in our patient suggests the potential presence of cell debris and an invisible tumor at the time of the initial surgery, contributing to implantation metastasis. Surgery has been shown to facilitate the growth of micrometastases at distant sites.17, 18 The resection of the primary tumor may disrupt the inhibitory control exerted by the primary tumor, known as “concomitant tumor resistance,” thereby slowing the growth of dormant metastases.18, 19 Additionally, the local extracellular environment, such as the rich vascular supply of scalp wounds, can provide essential nutrients and growth factors to disseminated tumor cells following surgical resection of a primary mass. Surgeons can take precautionary measures to minimize implantation metastasis risk during surgery, such as preserving tumor integrity, conducting piecemeal resection to prevent intraoperative tumor fragment dispersion, isolating equipment that has come into contact with the tumor to avoid cross contamination, and repetitively rinsing exposed tissues with saline.20
| Author, year | Age/Gender | Time interval (months) | Number of surgeries in total | Surgery for the metastasis (Y/N) | Adjuvant radiotherapy | Follow up (months) | Complications | Mortality |
|---|---|---|---|---|---|---|---|---|
| Darwish et al., 200428 | 53/F | 7 | 3 | Yes | Yes | NR | None | Alive |
| Ozer et al., 200729 | 45/F | 24 | 2 | Yes | Yes | 18 | None | Alive |
| Messerer et al., 200830 | 69/F | 41 | 3 | Yes | Yes | 12 | None | Alive |
| Velnar and Bunc, 200831 | 37/F | 120 | 2 | Yes | No | NR | NR | NR |
| Tahir et al., 200832 | 48/F | 18 | 2 | Yes | No | 24 | None | Alive |
| Enwereuzo, et al., 201833 | 56/F | 98 | 2 | Yes | No | NR | NR | NR |
| Nawashiro et al., 201924 | 79/M | NR | 2 | Yes |
GKRS 18 Gy |
30 | No | Alive |
| Liu et al., 202020 | 69/M | 72 | 1 | Yes | Yes | 6 | No | Alive |
- Abbreviations: GKRS, gamma knife radiosurgery; NR, Not reported.
The patient underwent surgery consistent with Simpson Grade I, involving excision of the skin patch, bone removal, and affected dura. Literature has consistently shown that complete tumor resection remains a significant prognostic factor, even when considering other variables like adjuvant radiation therapy and the updated WHO classification of 2016. The extent of resection (Simpson grading) remains a strong prognostic indicator, with Simpson Grade I resections yielding more favorable outcomes.21, 22 Recent evidence suggests the utility of 5-aminolevulinic acid (5-ALA) fluorescence-guided bone resection in infiltrating meningiomas, aiding in the identification of tumor remnants and reducing meningioma recurrence rates.23 Cranioplasty with a titanium mesh offers a stable and cosmetically pleasing solution for extensive bone defects, as seen in this patient with extensive osseous involvement following tumor surgery.
The literature review suggested a median interval of 41.50 months, with shorter intervals for higher-grade (WHO grade II and III) meningiomas, indicating earlier presentation of metastasis in high-grade cases.20 This underscores the importance of close follow-up for postoperative patients with WHO grade II and III meningiomas. Due to the scarcity of metastatic meningiomas, there are currently no established treatment protocols. The patient in this case underwent chemoradiotherapy postoperatively, consistent with the treatment approach in most of the reviewed cases. Gamma knife radiosurgery (GKRS) has been used successfully for the treatment of residual scalp metastasis, achieving complete recovery without neurological deficits or recurrence.24 GKRS has also demonstrated efficacy as a primary treatment modality for atypical meningiomas.25 Postoperative radiotherapy has been associated with improved local control, progression-free survival, and is considered an effective treatment for postoperative metastatic meningioma patients.26, 27
6 CONCLUSION
Scalp metastasis resulting from atypical meningioma is a rare clinical entity. The common mechanism of metastasis observed in reported cases is the implantation of tumor cells during surgical resection. Therefore, it is imperative for surgeons to adhere to meticulous intra and postoperative surgical practices to minimize the risk of tissue contamination.
AUTHOR CONTRIBUTIONS
Saad Javed: Conceptualization; data curation; writing – original draft. Amina Khan: Data curation; investigation. Ayesha Khalid: Data curation; formal analysis. Gianluca Scalia: Supervision; validation; visualization; writing – review and editing. Giuseppe Emmanuele Umana: Supervision; validation; visualization. Ashraf Mahmood: Methodology; resources; supervision. Eesha Yaqoob: Software; supervision; validation; visualization. Bhavya Pahwa: Supervision; validation; visualization. Bipin Chaurasia: Supervision; validation; visualization.
ACKNOWLEDGMENTS
None.
FUNDING INFORMATION
This manuscript did not receive any funds.
CONFLICT OF INTEREST STATEMENT
None.
ETHICS STATEMENT
This case report was compiled after obtaining informed consent from the patient for the disclosure of clinical history and management with the intention of publication. All attached imaging and clinical materials were de-identified to ensure patient anonymity.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated, or the article describes entirely theoretical research.




