Exercise-induced atrial fibrillation: A case report
Abstract
Key Clinical Message
Middle-aged male athletes, with or without underlying coronary artery disease, exhibiting exercise induced blood pressure (BP) variability and diabetes can have an increased risk of developing atrial fibrillation (AF). Assessment in athletes should include long-term arrhythmia monitoring. In addition, it is important to exert patients beyond their calculated target heart rate (HR) during an exercise stress test to detect exercise-induced AF. We suggest this strategy be specifically used for athletes with complaints of intermittent palpitation and chest pain. Referral to an electrophysiologist for a possible ablation procedure should be considered for the management of AF in athletes in whom the use of beta-blockers may limit exercise tolerance. Bleeding risk with the use of oral anticoagulation needs to be adequately evaluated in athletes with AF who engage in high-intensity exercise or activities.
The report highlights the case of a 54-year-old Caucasian male (height 5.11′, BMI 29.8) who presented with complaints of chest pain, mild coronary artery disease, palpitation, dizziness, and labile BP with high-intensity biking exercise. Diagnostic tests (exercise stress test, cardiac catheterization, Holter monitor, and Bardy patch) using standard procedure were unsuccessful at detecting the problem. In a repeat exercise stress test, the patient was exerted beyond the calculated HRmax (up to 117%) when the patient's heart rhythm flipped from sinus rhythm to AF. The patient was referred to a cardiac electrophysiologist and an ablation procedure was performed to prevent exercise-induced AF with high-intensity exercise. Young adults, with or without early coronary artery disease, performing high-intensity endurance exercises may be at risk of developing exercise-induced AF. This phenomenon is prevalent and well documented in the skiing population and patients with variance in BP during exercise. Endurance athletes tend to have a lower resting HR. As such, the use of standard rate-control medications in patients with exercise-induced AF may not be appropriate. Referral to a cardiac electrophysiologist and ablation procedures should be considered in this population for management and symptom control. If tolerated, especially in young adults with complaints of palpitation and chest pain, patients should be exerted beyond their calculated HRmax during an exercise stress test to diagnose an underlying condition of exercise-induced AF.
1 DETAILS OF THE CASE
A 54-year-old Caucasian male (height 5.11′, BMI 29.8) presented with complaints of intermittent experience of chest pain with exertion to our clinic in 2018. The patient reported exercising (biking) regularly at high intensity with no history of smoking, alcohol intake, or drug use. Chest pain generally (although not always) presented while biking. Previously, with a different practice, the patient had multiple evaluations for chest pain using exercise stress tests and echocardiograms which were normal. Patient was also started on antianginals. However, he did not tolerate Renexa experiencing severe shortness of breath and Imdur for severe headaches. With symptoms not being adequately controlled, the patient consulted us for a second opinion. Patient's past medical history includes hypertension, hyperlipidemia, gastrointestinal reflux disease, and diabetes mellitus type II. During this visit, patient did not complain of chest pain. His main concern was adequately controlling his blood pressure (BP) which was labile and fluctuated extensively before and after biking. Previously, patient failed to tolerate amlodipine, lisinopril, and hydralazine. Currently, BP was managed with losartan 25 mg QD and hydrochlorothiazide 25 mg QD. Due to the labile BP and hypertensive episodes during exercise, a renal ultrasound was ordered to evaluate renal artery stenosis which was negative.
Thereafter, in late December 2019, the patient presented to the clinic with complaints of experiencing chest pain again. Patient indicated that the chest pain was primarily experienced while biking and with BP spikes. His EKG showed sinus bradycardia (HR 50 bpm) with paroxysmal atrial contractions (PACs). With the patient being symptomatic, a nuclear (Cardiolite) Lexiscan stress test was ordered and completed in January 2020. The nuclear stress test was ordered considering a negative exercise stress test in the past. Results indicated small-sized mild severity anteroseptal completely reversible defect suggestive of ischemia in the territory of the mid and distal left anterior descending artery.
The positive stress test was followed by cardiac catheterization in February 2022 which showed nonobstructive coronary artery disease (20%–30% stenosis) without requiring any intervention. An echocardiogram done during this time showed ejection fraction was 55%–60% with mild left ventricular hypertrophy, normal sized left and right atrial, and ventricular chambers without any wall motion or diastolic dysfunction. Thyroid function was normal and a 48-h Holter showed a 2.2-s pause while sleeping, episodes of sinus tachycardia during exercise and occasional PACs, supraventricular couplets, and rare PVCs. One episode of supraventricular tachycardia (11 beats) was noted. The results did not indicate the need for any intervention. The patient was advised to follow up with his primary care provider for noncardiac-related chest pain. We also referred the patient to a pulmonologist to rule out any pulmonary etiology for chest pain.
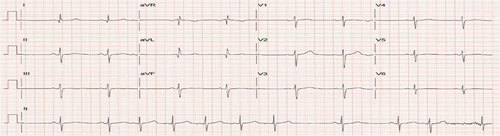
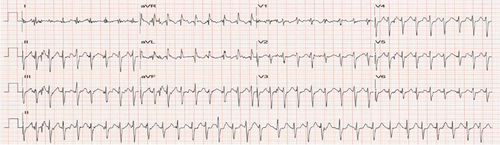
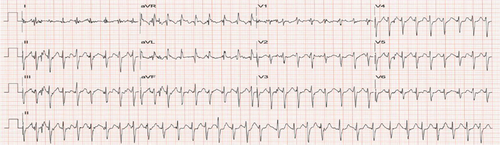
The patient followed up for a regular visit again in June 2022 and reported that he had remained asymptomatic and was overall doing well. He was staying active physically and had logged more than 1000 miles on his bike for the year. He had decreased the dosing of his BP medication as he was noticing his BP drop with extensive biking. In August 2022, the patient presented to the clinic with complaints of chest pain, palpitation and dizziness while biking. Patient reported incidents of both hypotension and hypertension while biking. Patient stated that he was experiencing “heart attack-like symptoms” when BP was elevated. When BP was low, patient felt weak and needed to stop biking. Patient was now apprehensive about taking BP medication for fear of dropping BP too low with exercise. To evaluate an underlying arrhythmia, a Bardy Patch for 2 weeks was ordered and the patient was asked to follow his normal routine. Additionally, a repeat treadmill exercise stress test was also ordered to assess BP response to exercise and look for arrhythmia induced by exercise. The patient had a difficulty wearing the Bardy Patch as the gel-based device would not stick on to the skin from excessive sweating while biking. For the Bruce treadmill stress test, the calculated target heart rate (HR) max was 166 bpm (220-age). During the test, the patient was monitored for patient-reported chest pain, breathlessness/exhaustion and not being able to keep up with test protocol, EKG changes specifically for ST changes or arrhythmias, and patient reaching HR reaching their 85% of target HRmax.1 HR was monitored using a 12-lead EKG and BP was checked at the end of each stage. Before starting the test, the patient's resting HR and BP were 63 bpm (sinus rhythm with PAC; Figure 1) and 136/82 mmHg. The patient achieved 100% target HR without any symptoms of chest pain or ST-T changes on the EKG (Figure 2). With the patient reporting no symptoms, feeling well and agreeing to continue exercising, the stress test was continued to Stage IV of the Bruce protocol. At this stage, when the patient had achieved 12 METS, peak HR was 195 beats/min (117% of target HRmax; Figure 3), BP was (180/86 mmHg), and the patient developed atrial fibrillation (AF). No ST-T changes were noted. The conversion of sinus rhythm to AF at high-intensity exercise correlated with the patient's report of experiencing palpitation, dizziness, and chest pain with high-intensity biking. The patient was diagnosed with exercise-induced AF.
2 DISCUSSION AND LITERATURE REVIEW
The prevalence of AF in the general population globally ranges between 2.1% and 3.4%.2-4 It is also the most commonly occurring arrhythmia among adults above 55 years. Additionally, studies using long-term monitoring devices have found a significantly higher proportion of older adults (>55 years) with no history of AF to have subclinical AF and be asymptomatic.5, 6 Ischemia from underlying coronary artery disease is an important risk factor for AF that needs to be ruled out using noninvasive (stress test, echocardiography, cMRI) and invasive (cardiac catheterization) procedures.
There is evidence to suggest that performing vigorous endurance exercise or sports for a prolonged length of time can be an independent risk factor for AF.7 In many cases, the odds of athletes developing lone AF can be high in athletes who have achieved a threshold of 2000 lifetime hours (OR 3.88, 95% CI: 1.55–9.73).8 Risk of AF in athletes performing high altitude sports such as skiing was positively correlated with number of years of endurance training and the number of races they competed in.9, 10 The risk may be 3- to 9-fold increase based on similar case-controlled studies to a 30% increase based on larger cohort studies.11 The risk may be present in both men and women.12
Various mechanisms have been suggested for the development of AF in athletes. Chiefly, atrial structural remodeling and enlargement in atrial size,13-15 atrial fibrosis from postexercise elevations in inflammation and oxidative stress especially with high-intensity exercise,16-18 autonomic influences altering vagal tone, and HR variability which may reduce refractoriness and enhance the excitability of the myocardium and favor reentry,19-21 and increased atrial ectopic triggers which is seen to be higher in runners with more than 4500 lifetime training hours.13 These mechanisms have been discussed in detail by Elliott et al.11 The development of AF at very high-intensity exercise may also be adrenaline induced. Prolongation in QTc interval and dramatic lowering of potassium levels has been observed in normal healthy adults with the infusion of adrenaline.22 Second, although not recorded during the stress test, there is a possibility that the patient may also be experiencing hypoxia-induced AF which matched reported symptoms of palpitation while biking. The possibility of hypoxia-induced AF has been previously reported in the literature.23 It is also likely that the patient's cardiac output was compromised during the AF episodes during exercise leading to the patient experiencing chest pain and dizziness. Additionally, abnormal BP response with exercise can be a risk factor for the development of paroxysmal AF.24 There is also evidence that high BP variability can be a risk factor for AF development in older adults and patients with diabetes or chronic kidney disease.25
The mainstay for the management of AF involves treatment for controlling ventricular rate (using nodal agents such as beta blockers) and/or rhythm (using antiarrhythmics), and anticoagulation for stroke prevention.26, 27 Additionally, in athletes, detraining is another strategy to reduce AF burden.11 Evidence shows that early rate control is associated with a lower risk of adverse cardiovascular outcomes.28 However, rate control for this patient using beta-blockers may not be the right option. First, patient's AF is paroxysmal in nature with a resting HR that is generally low (recorded between 47 and 66 bpm between 2017 and 2022). There is concern that the potential use of beta-blockers can exacerbate bradycardia, limit exercise tolerance, and can induce cardiogenic shock. Additionally, the use of rhythm control agents in endurance athletes with paroxysmal fib is not adequately studied.11 We consulted an electrophysiology cardiologist and in discussion with the patient, an ablation procedure was suggested for rate control. Ablation procedure in athletes has shown to be highly effective.29-31 Post ablation procedure in December 2022, the patient has remained in sinus rhythm. Patient continues to bike at high-intensity daily using a stationary bike without any complaints of palpitation, chest pain, and dizziness. He is continuing to experience bloop pressure fluctuations before (150/100) and after (120/80) exercise. Importantly, the patient has been asymptomatic, and the fluctuations are likely from the vasodilatory effect of exercise.32-34 The patient is on oral anticoagulation therapy. However, because of bleeding risks, the use of oral anticoagulation in young athletes engaging in high-intensity exercise or risky sports and activities needs careful consideration.
3 SUMMARY
As seen in our case, athletes, especially of male sex, middle aged and exhibiting exercise-induced BP variability, can have an increased risk of developing AF despite lower prevalence of conventional risk factors. Additionally, the patient also had risk factors of diabetes and mild nonobstructive coronary artery disease. It is likely that the AF was induced secondary to exercise-related myocardial ischemia. It is important to rule out AF secondary to underlying myocardial ischemia via stress test, echocardiography, cMRI, and cardiac catheterization if indicated. These standard diagnostic procedures, however, may not be adequate in detecting exercise-induced paroxysmal AF. We suggest that, if tolerated, especially in athletes with or without risk factors, patients be exerted beyond calculated HRmax during an exercise stress test to diagnose an underlying condition of exercise-induced AF. Referral to an electrophysiologist and ablation procedure to manage the condition should be considered.
AUTHOR CONTRIBUTIONS
Pallav Deka: Conceptualization; investigation; methodology; writing – original draft; writing – review and editing. Caitlin Mathison: Investigation; resources; writing – review and editing. George Abela: Validation; writing – review and editing. Milind Karve: Conceptualization; investigation; resources; supervision; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to disclose.
ETHICS STATEMENT
An IRB application was submitted to the Ehtics board at Michigan State University and written consent was obtained from the patient for publishing this case report.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Deidentified data will be made available upon reasonable request.




