Caseous mitral annulus calcification: A forgotten benign condition mimicking cardiac mass, a case report
Abstract
Key Clinical Message
Caseous mitral annulus calcification is a rare benign condition that can be misdiagnosed on echocardiography especially when it presents as a mass. This report highlights the contribution of cardiac MRI and computed tomography to the diagnosis through the case of a patient previously treated for breast cancer.
We report the case of a patient, previously treated for breast cancer, in whom echocardiography suggested the diagnosis of a cardiac tumor due the presence of a mass on the posterior mitral annulus. Cardiac magnetic resonance was inconclusive. Computed tomography confirmed the diagnosis of caseous mitral annulus calcification.
1 INTRODUCTION
Caseous mitral annulus calcification (CMAC) is an extremely rare condition, diagnosed in less than 1% of patients with degenerative MAC.1, 2 Its etiopathogenesis is controversial. Patients generally present with few clinical symptoms and it occurs mainly in old women.1 It may be mistaken for a cardiac tumor on echocardiography appearing as a mass with a central lucency and peripheral hypoechoic rim.3 Cardiac magnetic resonance (CMR) can be used for better characterization, but may be confusing. Computed tomography (CT) is the key diagnostic imaging technique. This report deals with the case of a relatively young patient in whom the diagnosis of CMAC was missed on echocardiography, doubtful on CMR and confirmed on CT by the presence of a mass-like calcification of the postero-lateral mitral annulus.
2 CASE HISTORY
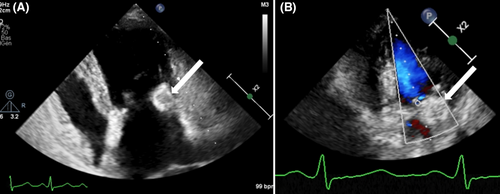
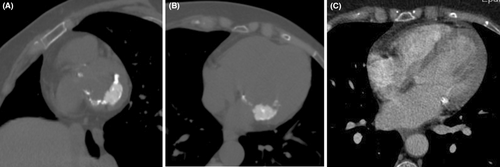
A 54-year-old woman was referred to the cardiology department for a transthoracic echocardiography (TTE) before undergoing general anesthesia for cataract surgery. She had been treated for invasive ductal carcinoma of the left breast, 8 years prior, receiving mastectomy, chemotherapy, trastuzumab, and radiotherapy. She had no personal or family history of cardiac diseases. The patient was asymptomatic and afebrile, and a systolic mitral murmur was identified upon clinical examination. Echocardiography showed a soft tissue mass with central echolucencies of the posterior mitral annulus blocking the posterior leaflet of the valve resulting in mitral regurgitation. There was no acoustic shadowing or blood flow on color Doppler (Figure 1). A diagnosis of a cardiac tumor, particularly a metastasis, was suggested. However, the patient refused to undergo a transesophageal echocardiography (TEE). Blood tests were normal with CA 125 within a normal range and no hyperleukocytosis or elevated C-reactive protein. The electrocardiogram was also normal. The patient underwent a CMR within a few days (Figure 2). Multi-planar cine images showed a low-signal mass of the posterior mitral annulus blocking the posterior leaflet and causing mitral regurgitation, extending to the myocardium. The mass was high-signal on T1 black blood weighted images and was low-signal on STIR weighted images. Late Gadolinium Enhancement (LGE) sequences showed peripheral intense enhancement. A cardiac CT with prospective gating and no contrast material administration showed that the mass was a huge calcification of the posterior mitral annulus with lower density than the other calcifications of the mitral and aortic annuli. Review of the radiological data of the patient at the work up of her breast cancer showed tiny calcifications of the mitral annulus (Figure 3). Cardiac tumor was eliminated and the diagnosis of CMAC was retained. The patient underwent her cataract surgery successfully and was followed after 6 months by her cardiologist with no clinical events.

3 DISCUSSION
Degenerative MAC is identified in 10.6% of the population with the caseous feature of calcifications being recognized in only 0.6%–0.7% on echocardiography.1.3. However, the prevalence of CMAC in autopsy series is higher (at around 2.7% of MAC cases), suggesting that this condition is often underdiagnosed on imaging and that patients are frequently asymptomatic.1, 3
It is frequently observed in elderly women with hypertension and dyslipidemia as well as in patients with kidney failure, in whom it is linked to calcium-phosphate metabolism deregulation.1, 4 However, the patient in our case is younger than those described in the literature, who are often over 60 years old. We believe that the age of occurrence and the size of the calcification can be explained by the radiotherapy she received which accelerated the process of mitral valve degeneration. Patients undergoing dialysis and presenting with this condition are also younger than others.5
CMAC is often an asymptomatic condition (as in the case we report) but patients can present with conduction abnormalities or with embolization of caseous material.3 CMAC is a dynamic process and can grow in size infiltrating the adjacent myocardium, as reported in our Case.6. The center of CMAC contains liquefied calcifications, cholesterol and fatty acids. Microscopic examination reveals a central amorphous acellular eosinophilic material with macrophages and lymphocytes as well as a peripheral fibrocalcic envelope.1, 6, 7 Despite these features, the caseous necrosis of MAC is not clearly explained.3
Multimodality imaging including echocardiography, cardiac CT and MRI, is mandatory in the work-up of a cardiac mass to avoid any unnecessary surgery.1 Pathological features can explain the radiological findings. On ultrasonography (TTE and TEE), CMAC has smooth borders, a semi-lunar shape a central echolucency with a hyperechoïc rim, a calcified envelope, no blood flow and no acoustic shadowing.1, 3, 7 CMR offers a better assessment and tissue characterization of the mass, which has two components. The center has a low signal on T2W and a higher signal than the myocardium on T1W images. The peripheral rim has a low signal on all sequences. First pass perfusion shows no enhancement whereas late gadolinium enhancement shows a peripheral enhancement.1, 7 However, MRI is not the best technique to detect calcifications whereas CT is. It shows a crescent-shaped mass in the atrio-ventricular junction that may extend to the atrium and/or ventricle with a center less hyperdense than the periphery.3, 7
Because of its scarcity, and variable appearance on ultrasound examination, CMAC is frequently confused with infective myocarditis vegetation, myocardial abscess, thrombus, and cardiac tumor.1, 3 In our case, endocarditis was ruled out as the mass was immobile and its appearance on ultrasound was different from vegetations. However, metastasis was feared because the patient had a history of treated breast cancer.
Cardiac tumors include benign and malignant primitive neoplasms as well as metastases. Primitive cardiac tumors are rare with an incidence varying between 0.002% and 0.3%.4 Benign tumors include myxomas, rhabdomyomas, papillary fibroelastoma, fibromas, hemangiomas, lipomas, and leiomyomas.8 Malignancy is suggested by rapid growth, invasion of adjacent structures, necrosis, hemorrhage, presence of feeding vessels, and involvement of more than one chamber.8 Primitive malignant cardiac tumors are extremely rare and are usually sarcomas. Angiosarcomas are the most frequent type in adults and typically arise in the right atrium. They have a poor prognosis with frequent metastatic spread.7-9 Metastases are much more frequent, found in 10%–12% of patients with a primitive cancer in post-mortem series.7 Primitive cancers that spread to the heart include lung cancer, breast cancer, lymphoma, and melanoma.7 In the case we presented, metastasis was among the suggested diagnoses due to the patient having been treated for breast cancer despite the long remission. Location, shape and tissue characteristics on multi-modality imaging can help distinguish CMAC from other pseudotumors and tumors (Table 1).7, 8
| Location | Shape | CT characteristics | MRI characteristics | ||||
|---|---|---|---|---|---|---|---|
| Cine views | T1 weighted imaging | T2 weighted imaging | Late gadolinium enhancement | ||||
| Pseudotumors | |||||||
| CMAC | Posterior and lateral left atrio-ventricular junction | Crescent-shape | Calcified with a center less dense than the peripheral rim | Immobile | Peripheral rim in low signal and core in high signal | Peripheral rim in low signal and center in low signal | Peripheral enhancement Dark avascular core |
| Abscess |
Atrio-ventricular junction Aorto-mitral courtain |
Spheroid | Peripheral enhancement with low density center | Immobile | Low signal core | High signal core | Peripheral enhancement |
| Thrombus | Any cardiac cavity | Spheroid | hypodense | Immobile | Low signal (depends on the age of the thrombus) | Hyposignal (depends on the age of the thrombus) | No enhancement |
| Benign tumors | |||||||
| Myxoma | Interatrial septum in the region of the fossa ovalis and develops in the left atrium | Polypoïd shape |
Hypodense Calcifications may be present |
Mobile | Low signal | High signal | Patchy |
| Fibroelastoma | Downstream valve | Polypoid | Hypodense | Mobile | Isosignal | Low signal | No enhancement or peripheral contrast material trapping |
| Fibroma | Myocardium | Spheroid | Isodense | Immobile | isosignal | Low signal | High contrast material uptake |
| Malignant tumors | |||||||
| Angiosarcoma | Right atrium | – | – | Immobile | Heterogenous | Heterogeneous | Heterogeneous |
| Rhabdomyosarcoma | Myocardium | – | – | Immobile | Isosignal | High signal | Homogeneous |
| Lymphoma | Right atrium | – | – | Immobile | Isosignal | Isosignal | No or minimal uptake |
| Metastasis | Any location | – | – | Immobile | Low signal | High signal | heterogeneous |
4 CONCLUSION
We reported the case of a patient who had a mitral mass on echocardiography suggesting a tumor However, CMR and cardiac CT ruled this out and the diagnosis of CMAC was finally retained. This condition is rare and very often asymptomatic, and it is frequently forgotten and misdiagnosed on ultrasound. Multislice imaging techniques, such as CMR and cardiac CT, offer a better assessment, show characteristic features and eliminate differential diagnosis including other pseudotumors as well as benign and malignant tumors.
AUTHOR CONTRIBUTIONS
Henda Nèji: Writing – original draft. Emna Bennour: Writing – original draft. Ines Baccouche: Investigation. Salma Kechaou: Investigation. Ikram Kammoun: Supervision; validation. Meriem Affes: Conceptualization. Saoussen Hantous-Zannad: Supervision; validation.
ACKNOWLEDGMENTS
A special acknowledgment to all contributors.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ETHICS STATEMENT
Written informed consent has been obtained from the patient.
CONSENT
The authors have confirmed during submission that patient consent has been signed and collected in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data of this case are available from the corresponding author upon reasonable request