A case of bladder and pelvic dead space inflammation successfully treated with endoscopic ultrasound drainage
Abstract
Key Clinical Message
Interventional endoscopic ultrasound (EUS) is effective not only for biopsy, but also for abscess drainage. We report the first use of EUS to drain inflammation of the bladder and pelvic dead space through the ileal conduit. EUS-guided drainage is effective in treating postoperative abscesses and should be employed more routinely.
The patient was a 77-year-old man with a vesicoureteral fistula. An ileal conduit was placed after abdominoperineal resection and partial bladder resection for local, postoperative recurrence of rectal cancer. During postoperative chemotherapy, the patient developed a high-grade fever and after a thorough examination, he was diagnosed with bladder and pelvic dead-space inflammation. All urine flowed through the ileal conduit, and it was assumed that secretions from the residual bladder and prostate gland had accumulated in the bladder and pelvic cavity, resulting in infection. A transcutaneous drain was inserted through the perineum and the infection was controlled, but it flared up again after the drain was removed. We concluded that long-term drainage was necessary and successfully controlled the infection by placing a plastic stent through the ileal conduit into the bladder and pelvic dead space under ultrasound endoscopy. This is the first report of ultrasound endoscopic drainage of an abscess through the ileal conduit.
1 INTRODUCTION
Inflammation of the pelvic dead space often occurs after abdominoperineal resection (APR) of the rectum.1 Usually, percutaneous drainage alone is sufficient to relieve symptoms, but sometimes treatment is difficult and may require a muscle cutaneous flap.2 In addition, a vesicourethral fistula after partial bladder resection at the time of APR usually does not resolve spontaneously and may require total cystectomy or urinary diversion.
In case of urinary tract injury, including various traumatic events or medical reasons, as well as for malignancy, simple repair should be considered first. However, sometimes challenging cases may require nephrectomy or cystectomy. Depending upon the patient's condition, urodynamic diversion with bladder preservation is sometimes chosen, but secretions from the residual bladder or prostate may cause dead space inflammation. In such a case, subsequent total cystectomy is too invasive, and percutaneous drainage is usually the treatment of choice.
Here, we present a successful case of dead space drainage using endoscopic ultrasound (EUS) from the ileal conduit after urodynamic diversion with bladder preservation. The ileal conduit is usually depressed into the pelvic cavity, which is in contact with the bladder/pelvic dead space. EUS-guided drainage through the ileal conduit is minimally invasive and extremely effective and it may be the first choice for percutaneous drainage.
2 CASE PRESENTATION
2.1 History of present illness
A 77-year-old male patient with rectal cancer underwent laparoscopic low anterior resection (D3 lymph node dissection, unilateral therapeutic lateral lymph node dissection, and right internal iliac artery dissection) after neoaduvant chemotherapy (four courses of FOLFOX), and was diagnosed with pT3 N3(#263Prt) M0, pStage IIIC. The patient was treated with adjuvant chemotherapy (oral UFT/LV for 6 months) and was followed-up. Unfortunately, there was a local recurrence in the anastomosis region, requiring laparoscopic APR (combined resection of the right wall of the bladder), and R0 resection was achieved. However, no urine was observed in the Foley catheter after the night of the surgery, and discharge from the abdominal drain was urine. On the third postoperative day, the perineal wound had separated and a vesicourethral fistula had formed. The patient was treated conservatively for about 3 months, but the fistula did not close. Finally, the fistula was filled with a bilateral gluteal muscle flap, but the vesicourethral fistula did not close, and the patient was finally cured after urinary diversion through an ileal conduit (Figure 1). Six months after the second surgery, tumor markers were elevated, and single liver and lung metastases appeared. Systemic chemotherapy (FOLFOX/bevacizumab) was initiated. One week after the second course of chemotherapy, the patient presented to our department with a high fever. After a thorough examination, he was diagnosed with bladder and pelvic dead space inflammation and was admitted to the hospital for further examination and treatment.
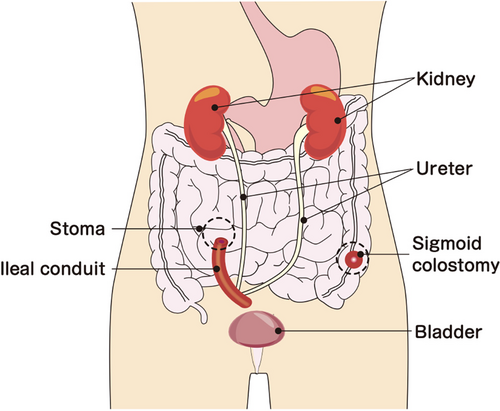
Past medical history: End-stage renal insufficiency (on hemodialysis), bilateral common iliac artery aneurysms.
Medications: Precipitated calcium carbonate, dimethicone.
Physical examinations: slight, acute distress; body temperature: 38.5; blood pressure: 88/47 mmHg; heart rate: 107/min; respiratory rate: 22/min, SpO2 99% (room air).
2.2 Laboratory data
Complete blood count: leukocytes 26,950/mL, hemoglobin 12.6 g/dL, platelets 196,000/mL.
Biochemistry: total protein 5.8 g/dL, albumin 2.9 g/dL, urea nitrogen 34.2 mg/dL, creatinine 3.03 mg/dL, CRP 13.47 mg/dL.
Tumor markers: CEA 7.7 ng/mL, CA19-9204.5 U/mL.
2.3 Post-hospitalization progress
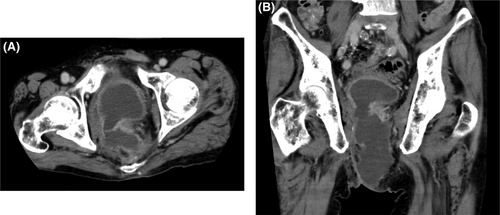
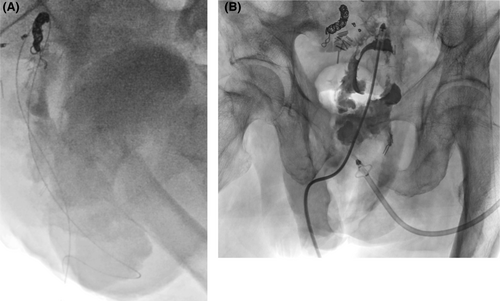
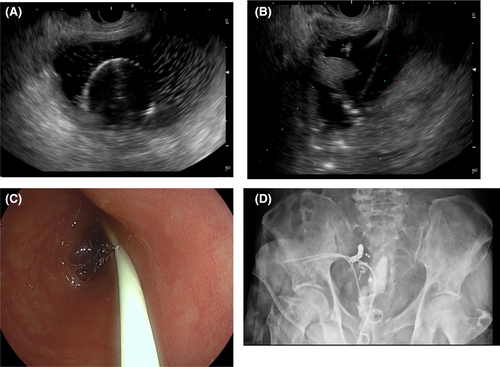
Contrast-enhanced computed tomography (CT) findings on admission (Figure 2) showed fluid retention in the bladder and similar fluid retention in the pelvic fistula as well. On the day of admission, transperineal percutaneous drainage was performed. Approximately 250 mL of purulent drainage fluid was recovered with continuous drainage. Inflammatory findings improved, and the drain was removed. However, the day after drain removal, the patient developed a high-grade fever again, and a CT scan revealed a flare-up of bladder and pelvic dead-space inflammation (Figure 3). Percutaneous drainage was performed again. Since long-term drainage was thought to be necessary, some kind of external fistula was considered. The ileal conduit was close to the bladder and abscess cavity, and it could be punctured safely with close attention to the urethral anastomosis. This procedure and associated risks were fully explained, and informed consent was obtained, as well as permission from the institutional review board. First, we approached the abscess cavity. An ultrasound endoscope (EUS, EG-740UT, Fujifilm) was inserted into the ileal conduit, and a 19G puncture needle (Expect, Boston Scientific) was used. A 0.025-inch guide wire (VisiGlide2, Olympus) was inserted, and the fistula was dilated with a 6-mm bile duct dilation balloon (Ren, Kaneka Medics), after which a 7Fr pigtail endoscopic retrograde biliary drainage tube (Advanix-J, Boston Scientific) was inserted. Next, the bladder was also approached as described above. A 7Fr energetic dilator (Fine025, Medicos-Hirata) was used to dilate the fistula, and a 7Fr pigtail ERBD tube (Advanix-J) was inserted. The ERBD tube was shortened so that the distal end was positioned at the stoma opening of the ileal conduit (Figure 4). Immediately after surgery, retrograde urine flow was observed from the perineal drain via the ileal conduit, but the drainage volume was gradually decreased, and the perineal drain was removed about 2 weeks later. Thereafter, inflammation did not recur. Two courses of chemotherapy (FOLFOX/bevacizumab) were added, and laparoscopic radiofrequency ablation was performed for the single liver metastasis, and thoracoscopic partial lung resection was performed for the lung metastasis. The patient is currently under observation without chemotherapy. Tube exchange was attempted 3 months after the first puncture. The tube in the ileal conduit could be replaced only by guidewire manipulation after slightly withdrawing the tube under the endoscope. Six months have passed since the puncture, and the patient is alive without recurrence.
3 DISCUSSION
In recent years, development of EUS intervention has been remarkable, and several approaches are now possible for various gastrointestinal tracts. Especially in surgical departments, EUS is considered very useful for drainage of postoperative intra-abdominal abscesses. Initially, percutaneous drainage was the only choice for a pancreatic fistula after gastrectomy or pancreatectomy, but the length of time that the tube was in place (several months or more) caused a decline in patient quality of life. Currently, we use transgastric or transenteral EUS drainage as the first choice.3-5 However, there are various issues to be considered, such as how soon after abscess formation the procedure should be performed, whether plastic or metal stents should be used, and whether internal fistula or naso-external fistulae should be used. For example, retrograde infection occurred during transgastric drainage for postoperative pancreatic fistula (using a 7Fr double pigtail) on the 10th day after pancreaticoduodenectomy, and the patient was fasted and treated with antimicrobials for about 1 week. Retrograde infection was thought to have occurred because the abscess was not sufficiently localized, and inflammation spread outward because of the early postoperative period. Although the technique has become common, there are no protocols with solid evidence.
This is the first case report of drainage of an abscess through the ileal conduit after urinary tract diversion. A search of PubMed in May 2023 for the search terms “ileal conduit” and “EUS” revealed only one case of endoscopic tumor biopsy through the ileal conduit.6 Although there have been many reports of transrectal drainage of pelvic abscesses7 since Giovannini et al.,8 the present patient does not have a rectum due to APR. Although puncture is possible if the elevated sigmoid colostomy is in contact with the abscess, there is a risk of retrograde leakage of fecal fluid into the peritoneal cavity, resulting in serious peritonitis. In this case, the ileal conduit was in contact with the abscess cavity of the bladder and pelvic dead space because the ileal conduit was used for urinary diversion. Since the ileal conduit contained urine, we assumed that it could be punctured, because this would not cause a serious problem even if it leaked into the abdominal cavity. However, the ureteral anastomosis had to be identified, and the patient could not be punctured near the anastomosis. (This case was treated successfully, perhaps because all procedures were performed in the surgical department, rather than in multiple departments. All surgical staff understood the postoperative anatomy and the procedure, so that we achieved the best outcome.) Since this was a pioneering case, the procedure and risks were fully explained to the patient and his family, and informed consent was obtained.
There are numerous cases in which bladder-preserving urinary diversion is performed for cancer treatment,9, 10 traumatic injury,11 surgical injury,12 or other reasons. In this case, the patient had cancer, had undergone multiple rounds of chemotherapy, and had undergone concomitant resection of the internal iliac artery at the time of the initial surgery, resulting in decreased blood flow and IgA production in the bladder. In such cases, an external fistula (vesicostomy or perineal drainage)13 or bladder balloon placement may be necessary, obviously degrading quality of life compared to an internal fistula. In the present case, a puncture was performed through the ileal conduit due to the absence of a rectum, but transrectal drainage should be considered in cases where the rectum remains. In this case, a plastic stent was used because there was a risk of retrograde leakage of urine, and also because of concerns about ileal conduit problems (e.g., bleeding, stenosis, or perforation due to abrasion) or migration of a metallic stent. Although periodic replacement is necessary, as in this case, a plastic stent can be easily replaced over a guidewire, and the overall advantage of the plastic stent is significant.
4 CONCLUSION
We performed transendoscopic drainage through the ileal conduit for a patient with bladder and pelvic dead space inflammation. We hope that more cases of transgastrointestinal drainage will be reported in the future, so that protocols may be established, including devices to be used and the timing of puncture.
AUTHOR CONTRIBUTIONS
Kazuhiro Hiyama: Conceptualization; investigation; methodology; resources; writing – original draft. Izumi Kirino: Writing – review and editing. Yasuo Fukui: Writing – review and editing.
ACKNOWLEDGMENTS
We are grateful to Takashi Hosokawa of Hosokawa Design Office for creating figure 1, as the authors indicated.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest and that there are no relevant financial disclosures to report.
ETHICS STATEMENT
This treatment strategy was approved by the Institutional Review Board of Atago Hospital.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the journal Editor-in-Chief on request.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.




