Persistently high serum aspartate aminotransferase level in an asymptomatic young patient
Xiong Pei and Yanhua Zhao contributed equally to this work.
Abstract
Key Clinical Message
We report a young man with isolated elevated AST. He had no other evidence of liver or other related diseases. All the tests and examination reports were negative. The final diagnosis of macro-AST was confirmed by PEG precipitation tests.
Elevated liver enzymes, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST), may commonly indicate liver injury. However, macro-AST is generally a benign condition that may be considered as pathologic by clinicians. A young man with isolated elevated AST for more than 10 years who have taken extensive tests and examinations was diagnosed with macro-AST in our article. Thus, in patients with isolated AST-elevation, polyethylene glycol (PEG) precipitation test was recommended to test whether macro-AST could be diagnosed.
1 INTRODUCTION
Macroenzymes, high molecular mass complexes of plasma enzymes with immunoglobulins (IgG, IgA, or IgM), are rarely detected in the general healthy population.1 Elevated enzyme activities in plasma may sometimes attribute to the presence of macroenzymes, which reduce plasma clearance and prolonged half-life.2
Macro-aspartate aminotransferase (macro-AST) is a typical macroenzyme that is generally characterized by increased serum AST activity.2, 3 While elevated serum AST activity is indicated as the sign of chronic liver disease, cardiac or skeletal abnormalities, macro-AST is generally a benign condition.4 However, clinicians usually erroneously consider this condition as pathologic, leading to unnecessary medical tests and even invasive liver biopsies, before the final diagnosis.5 There are many different methods for detecting macro-AST, including gel filtration chromatography (GFC), ultracentrifugation (UF), polyethylene glycol (PEG) precipitation, and refrigeration at 4°C.3, 6
We report a case of an asymptomatic patient with persistent isolated elevation of AST for 10 years. The presence of macro-AST was finally confirmed by the PEG precipitation method, which also showed the refrigeration method as invalid.
2 CASE PRESENTATION
2.1 Patient
A 32-year-old male (height: 1.72 meters, BMI: 24.2) was admitted to our hospital's infectious disease department because of isolated elevated AST activity over the past 10 years. He was asymptomatic with a normal physical examination. Only minor depression was mentioned in his medical history from the past year, for which he was treated with paroxetine hydrochloride tablets, 10 mg once a day. He denied using over-the-counter, traditional Chinese medicines, and alcohol consumption. Informed consent was obtained from the patient.
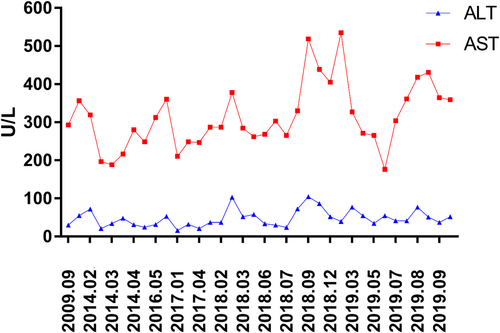
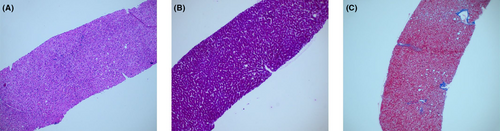
During a health examination 10 years ago, an isolated elevation of the AST (293 U/L) with normal alanine aminotransferase (ALT) was first observed in this patient. Since then, several liver function tests confirmed the isolated elevation of the AST, with values in the range of 177–535 U/L (normal range: 10–50 U/L) (Figure 1). All laboratory-related investigations were mostly normal during this time, including ALT, lactate dehydrogenase, alkaline phosphatase, gamma-glutamyltransferase, creatine kinase, total bilirubin, conjugated bilirubin, thyroid stimulating hormone (TSH), free thyroxine (FT3), free thyroxine (FT4), and prothrombin time. Viral serological markers for hepatitis A, B, C, D, and E and tests for the cytomegalovirus and Epstein–Barr virus were negative. Tests for metabolic liver diseases (α-1-antitrypsin, ceruloplasmin, ferritin, and serum iron) and autoimmune liver diseases (antinuclear, antimitochondrial, anti-smooth muscle, and anti-liver-kidney microsomal) were negative. A liver ultrasound indicated mild infiltration of fat, quantitated as <11% steatosis by the controlled attenuation parameter (CAP) test of 124 dB/m (normal limit < 238 dB/m). Liver biopsy findings suggested mild steatosis (less than 5%), and no fibrosis or iron overload was found (Figure 2).
Tests to detect cardiac function, such as electrocardiogram, ultrasonic cardiogram, and cardiac biochemical markers, did not show any abnormalities. High resolution CT of the chest and abdominal ultrasound were used to exclude the causes of elevated AST activity from lungs, pancreas, kidneys, and spleen. Additionally, glucose metabolism and lipid metabolism showed no abnormalities.
2.2 Laboratory tests for the diagnosis of macro-AST
2.2.1 PEG precipitation tests
Equal volumes (200 μL) of a 25% solution of PEG 6000 and the serum of patients and six control samples were vortex mixed for 1 min, equilibrated for 10 min, and centrifuged at 3000 rpm for 10 min. The resulting clear supernatant was used for the quantitative determination of AST (AST PEG). Simultaneously, 200 μL of the serum of patients and the six control samples were mixed with 200 μL physiological saline solution for measuring AST (AST DIL). The effect of the PEG precipitation test is determined by the percentage of AST recovered after precipitation (AST recovery %), which is measured by 100 × [AST PEG/AST DIL], and the percentage of PEG precipitation activity (PPA) is measured by 100 × [(AST DIL-AST PEG)/AST DIL].3 The cut-off point of PPA > 73% was considered positive and PPA < 48% was considered negative, which were the reference standards for diagnosing macro-AST.3
The patient's original AST activity was 364.7 U/L. AST activity decreased to 28.2 U/L after precipitation with PEG, with the AST recovery of 15.44% and PPA of 84.56%. In the six control samples, AST recovery was above 67% (range 67.10%–81.97%) and PPA was below 32% (range 18.06%–32.95%) (Table 1). According to the reference standard, PEG precipitation confirmed the presence of macro-AST in the patient's serum.
| AST(U/L) | AST (U/L) | AST DIL½ (U/L) | AST DIL½ (U/L) | AST PEG(U/L) | AST PEG(U/L) | AST recovery (%) | AST recovery (%) | %PPA | %PPA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Original serum | Frozen serum | Original serum | Frozen serum | Original serum | Frozen serum | Original serum | Frozen serum | Original serum | Frozen serum | |
| Patient | 364.7 | 361.5 | 182.6 | 180.8 | 28.2 | 26.5 | 15.44 | 14.66 | 84.56 | 85.34 |
| Control 1 | 109.0 | 109.6 | 56.5 | 57.2 | 44.2 | 43.7 | 78.23 | 76.40 | 21.77 | 23.60 |
| Control 2 | 277.0 | 275.4 | 143.4 | 141.5 | 117.5 | 116.1 | 81.94 | 82.05 | 18.06 | 17.95 |
| Control 3 | 390.0 | 393.7 | 201.5 | 199.8 | 135.1 | 132.3 | 67.05 | 66.22 | 32.95 | 33.78 |
| Control 4 | 449.0 | 450.8 | 227.4 | 227.0 | 184.5 | 186.1 | 81.13 | 81.98 | 18.86 | 18.01 |
| Control 5 | 723.0 | 719.3 | 378.4 | 363.9 | 253.9 | 249.7 | 67.10 | 68.61 | 32.90 | 31.38 |
| Control 6 | 922.0 | 915.1 | 487.7 | 479.3 | 395.8 | 392.5 | 81.16 | 81.89 | 18.84 | 18.10 |
- Abbreviations: AST DIL, AST activity diluted with physiological saline solution; AST PEG, AST activity by precipitation with polyethylene glycol; AST, aspartate aminotransferase; %PPA, the percentage of PEG precipitation activity.
To evaluate the stability of the sample, frozen aliquots from patient serum and six controls were stored at −20°C for 7 days. Similarly, the AST activity of the patient's sample gave an AST recovery of 14.66% and PPA of 85.34%. This result indicated greater precipitation than the control samples, which had AST recovery above 66% (range 66.22%–82.05%) and PPA below 33% (range 33.78%–17.95%) (Table 1).
2.2.2 Refrigeration stored at 4°C
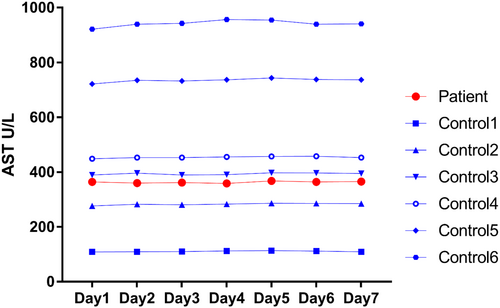
To evaluate the degradation of the enzyme, AST was determined in the patient and six control samples every 24 h for seven consecutive days of refrigerated storage (4°C).
As shown in Figure 3, AST activity in the serum of patient and six controls remained at the same level after stored at 4°C for 1 week. To confirm this result, we repeated the procedure with new sera obtained from the patient and obtained similar stabilities in the results (Table 2).
| AST(U/L) | AST(U/L) | Variation (%) | |
|---|---|---|---|
| Original serum | Stored serum | ||
| Patient first | 431.0 | 430.1 | 0.21 |
| Patient second | 364.7 | 365.9 | 0.32 |
| Patient third | 359.0 | 357.9 | 0.30 |
- Abbreviation: AST: aspartate aminotransferase.
2.2.3 DNA isolation and sequencing
Serum AST activity is predominantly of cytoplasmic origin and is encoded by glutamate oxaloacetate transaminase 1 (GOT1) on chromosome 10q24. A missense variant in GOT1 (p. Gln208Glu, rs374966349) is thought to be a causal variant in familial macro-AST.7 To detect the GOT1gene, fresh blood from the patient was sent to Genesky Biotechnologies Inc., Shanghai. The sequencing results showed that no variants were discovered in the coding regions of the GOT1 gene.
3 DISCUSSION
Our study reported a man with a history of elevated AST activity for almost 10 years who was finally diagnosed with macro-AST. He was asymptomatic and had no other indicators for liver or other diseases to account for the isolated elevated AST activity. The final diagnosis was confirmed by PEG precipitation.
Macro-AST was first reported in 1978 by Konttinen A et al. in two healthy young women with elevated serum levels of AST.8 After elevated serum AST activity is excluded as the sign of chronic liver disease, cardiac or skeletal abnormalities, macro-AST should be considered.4 Subsequently, many cases on macro-AST have been published. Macro-AST has been reported in some patients with allergic rhinitis, chronic hepatitis C, multiple myeloma, medium-chain acyl CoA-dehydrogenase deficiency, and non-alcoholic steatohepatitis cholecystolithiasis.2, 5, 9-12 We conducted a thorough review of the articles about macro-AST cases over the past 5 years. We found that macro-AST could happen in any ages. Most cases were asymptomatic and only had a history of elevated AST activity. Almost all of the cases were diagnosed by PEG precipitation (Table 3).2, 3, 13-19
| Age, sex, country | AST level | Period of isolated elevation AST | Negative | Positive | Diagnosis method |
|---|---|---|---|---|---|
| 72, female, Spain13 | 183 IU/mL | August 2020–April 2021 | Serology, thyroid hormones, ceruloplasmin, and alpha-1-antitrypsin | Stage 1 chronic kidney disease, steatosis liver, antinuclear antibodies (1/640 spotted pattern and 1/1280 MID-BODY pattern) | PEG precipitation |
| 50, female, Spain3 | 368–532 U/L | NA | Hepatitis, cytomegalovirus and Epstein–Barr virus, autoimmune hepatitis, Wilson's disease, hemochromatosis, myopathies, celiac, and thyroid diseases | None | PEG precipitation, Stored at 4°C |
| 48, male, USA14 | 212–518 U/L | December 8, 2011–November 9, 2021 | Viral hepatitis, autoimmune hepatitis, ceruloplasmin, alpha-1-antitrypsin, ferritin level, and creatinine kinase | Mild liver steatosis (ultrasonography) | PEG precipitation |
| 22, female, Japan15 | 232 U/L | 2 years | HBV, HCV, cytomegalovirus, Epstein–Barr virus, connective tissue diseases, and metabolic diseases | None | Stored at 4°C |
| 18 months, boy, Iran16 | 198–351 U/L | 3 years | TSH, HBV, HCV, ANA, Anti LKM, anti smooth muscle Ab, Anti TTG IgA, ceruloplasmin, Alfa 1 antitrypsin | Kawasaki disease | PEG precipitation |
| 46, female, USA2 | 136–759 U/L | 2006–2019 | Hemolysis, viral hepatitis, autoimmune hepatitis, alpha-1 antitrypsin, ferritin levels, creatine kinase, and aldolase | NAFLD (liver biopsy) | PEG precipitation |
| 34, female, China17 | 76.2–170 U/L | 7 months (April 10, 2018-September 11, 2018) | Viruses, ceruloplasmin, iron concentrations, thyroid function, Anti-dsDNA, Anti-M2, Anti-gp210, Anti-SP100, Anti-SSA-52/Ro52, Anti-Sm, Anti-nRNP/Sm, and Anti-LKM | Mild cholecystitis (abdominal ultrasonography) | PEG precipitation |
| 55, female, USA18 | 150–250 U/L | NA | Viral hepatitis (HAV, HBV, HCV), Autoimmune serologies (ANA, Alpha-1 Antitrypsin, Actin IgG Antibody, Cyclic Citrullinated Peptide IgG Antibody, Anti-M2, Anti-Sm, Histone Antibody, Anti-rRNP, anti-DNA-IgG antibody, RF, anti-MPO Antibody, Proteinase-3 Antibody, SSA/SSB, tTG Antibody, Aldolase | Gastroesophageal reflux disease, obstructive sleep apnea, obesity, asthma, and depression | PEG precipitation |
| 28, female, Germany19 | 386–505 U/L | 12 months | Hepatitis A, B, C, D and E, ferritin and serum iron levels, anti-nuclear antibodies, and anti-mitochondrial antibodies | None | PEG precipitation |
There are many different methods for detecting macro-AST, including GFC, UF, PEG precipitation test, and stored refrigeration at 4°C.3, 6 The final diagnosis of macro-AST was confirmed by the PEG precipitation test.3 GFC and UF require highly specialized equipment and are time-consuming, making their unavailability in most clinical laboratories. The PEG precipitation test is simple and inexpensive and has been considered as a standard for the diagnosis of macro-AST.7, 20 It was reported that 73.3% PPA corresponds to 88.2% sensitivity and 88.9% specificity.20 In our case, the original AST activity was reduced by about 92% in the patient's sample after precipitation with PEG. The PPA of 84.56% suggests that a high level of sensitivity and specificity was likely.
It has been reported that if AST activity decreases more than 65% after samples are stored in the refrigerator (2–8°C) for 2–7 days, the diagnosis will probably be that of macro-AST.3, 5, 21 However, the AST activity in our patient's serum remained at the same level after stored at 4°C for 1 week, as well as that for the six controls. Chtioui et al. also found no significant change of AST activity in serum stored at 4°C.4 This discrepancy may be due to the different types of interacting immunoglobulin, plasma, and other proteins.4
Macro-AST is a rare, but benign disease, with a prevalence of 0.014% and 9.09% among general gastroenterological patients and those with isolated elevated AST without liver abnormalities, respectively.7 Serum AST activity is influenced by both genetic and environmental factors.22 A rare in-frame deletion of three nucleotides in GOT1, encoding asparagine at position 389 (p.Asn389del), leads to decreased AST activity levels.22-24 From the results of whole-exome sequencing-based screening in a large cohort of macro-AST patients, the GOT1 p.Gln208Glu mutation was detected in 50 (54.3%) of 92 probands from 20 of 29 (69%) families,7 indicating that a genetic test for this variant may aid the diagnosis of macro-AST. However, we did not find a GOT1 gene mutation in our patient. More studies should be conducted to explore these results in the future.
4 CONCLUSION
Macro-AST is a rare, but benign disease in clinical practice. If patients present with isolated AST-elevation, PEG precipitation tests should be performed to diagnose macro-AST. Early recognition of macro-AST could avoid extensive or invasive investigations and treatments.
AUTHOR CONTRIBUTIONS
Xiong Pei: Writing – original draft. Yanhua Zhao: Writing – original draft. Wei Jiang: Writing – review and editing. Qingmin Zeng: Writing – review and editing. Changhai Liu: Writing – review and editing. Ming Wang: Writing – review and editing. Hui Wang: Writing – review and editing. Wei Gan: Writing – review and editing. Shanshan Liang: Writing – review and editing. Dongbo Wu: Conceptualization; project administration; supervision. Hong Tang: Conceptualization; project administration; supervision.
ACKNOWLEDGMENTS
The authors would like to be grateful to the patient for participation in the current study.
FUNDING INFORMATION
This research was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYGD20009), the Science and Technological Supports Project of Sichuan Province, China (No. 2022YFS0338), Natural Science Foundation of Sichuan Province (No. 2022NSFSC0732), Post-Doctor. Research Project of West China Hospital of Sichuan University (No. 2020HXBH079).
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest to be declared.
CONSENT
Written informed consent was obtained from the patient.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of the study are available upon request.