Enhanced Precision in the Convergent Procedure for Persistent Atrial Fibrillation Using Real-Time Intraoperative 3D Electrophysiological Mapping
Funding: The authors received no specific funding for this work.
ABSTRACT
Real-time intraoperative electrophysiological mapping significantly enhances the precision and efficiency of ablations in epicardial ablation (Convergent procedure) for persistent atrial fibrillation. This novel approach improves patient outcomes by enabling targeted ablation of affected tissues, reducing unnecessary burns, and ensuring discharge in sinus rhythm, as we demonstrate.
1 Introduction
Atrial fibrillation (AF) is widely recognized as the most prevalent sustained arrhythmia, affecting approximately 5 million adults in the United States alone [1]. However, since most individuals with AF remain asymptomatic and undiagnosed, the actual prevalence is likely considerably higher. As the global population ages, the incidence of AF is projected to rise over time, with over 12 million adults anticipated to be affected by AF in the United States alone by 2030 [2]. Although patients with AF often experience no symptoms, AF carries a significant morbidity and mortality burden. In 2019, 183,321 death certificates in the United States attributed AF as a contributing factor, with 14.5% directly linked to it [3]. Furthermore, AF contributes to the financial strain on the United States healthcare system, costing approximately $34 billion in the 2021–2022 financial year, a figure that will only increase with an aging population [4].
AF is a condition characterized by the formation of abnormal electrical foci that disrupt the normal depolarization of the atria. Although the precise pathophysiology underlying these abnormal foci remains unclear, it is believed that atrial myocardial stretch, inflammation, and scarring play a role. Additionally, the metabolic, electrical, and mechanical remodeling of the left atrium, often accompanied by a degree of heart failure, can further exacerbate AF. Notably, 75% of individuals with AF experience non-paroxysmal AF, which includes persistent (25%) and permanent (50%) episodes [5]. This prevalence significantly increases the risk of major adverse cardiovascular events, such as stroke, ultimately diminishing quality of life and life expectancy [6].
A diverse array of interventions exists for AF, ranging from pharmacological therapies (such as anti-arrhythmic or negative chronotropic medications) to ablations, which aim to interrupt aberrant conduction pathways. Ablations may be catheter-based, surgical (“Maze” procedure), or through a hybrid approach. Catheter-based radiofrequency ablation (RFA) frequently serves as a primary treatment option and typically achieves a high success rate for rhythm control in paroxysmal AF [7]. However, this approach is less effective for persistent or permanent AF due to cardiac remodeling and alterations in electrical pathways and ion channels [8]. The hybrid “Convergent” procedure constitutes a treatment strategy that integrates combined epicardial and endocardial ablation techniques. Typically, epicardial surgical ablation is performed initially, followed by catheter-based endocardial ablation approximately 1–3 months later to ensure durable transmural lesion sets. The CONVERGE trial demonstrated the superiority of hybrid ablation over catheter ablation, with over 90% of patients in the hybrid group achieving freedom from AF at 18 months [9].
Intraoperative three-dimensional (3D) electrophysiological (EP) mapping employing novel systems, such as the EnSite Precision system (Abbott Inc., Chicago, IL), provides a more informative and precise approach to the Convergent procedure. The mapping system generates a map based on readings from the probe as it traverses the epicardial surface. Residual signals detected after ablations enable confirmation of complete electrical silence. Although its efficacy is not yet fully documented, real-time intraoperative 3D EP mapping in Convergent procedures holds the potential to enhance ablation precision and improve outcomes in persistent AF patients by guiding specific ablation at sites of disorganized electrical activity and verifying electrical silence post-ablation. We present the successful utilization of intraoperative 3D EP mapping using the EnSite Precision system before and following each burn during a hybrid Convergent procedure for a patient with persistent AF.
2 Case Presentation
A 78-year-old man was referred to our center for surgical evaluation due to long-standing, persistent AF refractory to medical therapy and 2 prior catheter ablations. He had been experiencing AF for 5 years and was referred by his cardiologist for cardiothoracic surgical consideration. The patient complained of chronic dyspnea at rest, which was exacerbated by exertion. He reported becoming winded during activities such as walking or performing activities of daily living (ADLs). His dyspnea was severe to the point of near syncope. He denied any associated chest pain, lower extremity edema, light-headedness, dizziness, or syncope episodes. There were no reported modifying factors. Despite his symptoms, the patient was motivated to undergo treatment to regain his previously active lifestyle. He expressed a strong desire to return to his baseline level of physical activity.
The patient had a complex medical history, including prostate cancer treated with radiotherapy, supraventricular tachycardia, hypertension, hyperlipidemia, and hypothyroidism. He also had a history of a pancreatic cyst, which was evaluated with endoscopic ultrasound and needle aspiration. The cyst was found to be a pseudocyst, requiring no further treatment.
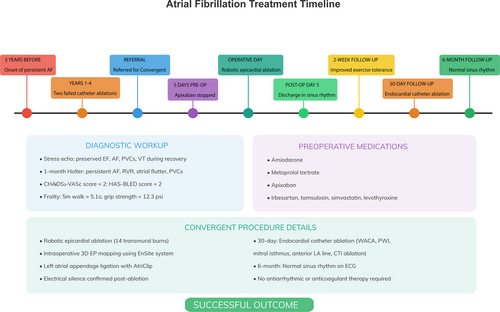
Preoperatively, the patient was maintained on a tailored pharmacological regimen aimed at managing persistent AF, hypertension, and associated cardiovascular risk factors. For rhythm control of his persistent AF, the patient had been prescribed amiodarone at a dose of 200 mg daily. This Class III antiarrhythmic was initiated following unsuccessful catheter ablation procedures and was selected for its efficacy in maintaining sinus rhythm in refractory AF, despite its known potential for systemic adverse effects. For rate control, the patient was taking metoprolol tartrate at 25 mg twice daily. This cardioselective beta-blocker was used to reduce the ventricular response rate during episodes of AF. The patient was also anticoagulated with apixaban, 5 mg twice daily, based on a CHA2DS2-VASc score of 2, indicating a moderate thromboembolic risk. Apixaban was chosen over warfarin for its favorable safety profile, including a lower risk of intracranial bleeding and no requirement for regular INR monitoring. Hypertension was managed with irbesartan at a dose of 150 mg daily. This angiotensin II receptor blocker was selected for its antihypertensive efficacy and potential benefits in mitigating atrial structural remodeling, which is particularly relevant in the context of AF. Other chronic medications included tamsulosin 0.4 mg daily for benign prostatic symptoms, simvastatin 20 mg daily for hyperlipidemia, and levothyroxine 50 μg daily for hypothyroidism. A summary of the key clinical events is given in Table 1.
| Timepoint | Event |
|---|---|
| 5 years before referral | Onset of persistent atrial fibrillation (AF) |
| Years 1–4 | Two failed catheter ablation attempts |
| At referral (0 months) | Referred for Convergent procedure due to refractory AF |
| Diagnostic workup | Stress echocardiogram: preserved EF, AF, PVCs, VT during recovery |
| 1-month Holter: persistent AF, RVR, atrial flutter, PVCs | |
| CHA2DS2-VASc score = 2; HAS-BLED score = 2 | |
| Frailty assessment: 5 m walk = 5.1 s; grip strength = 12.3 psi | |
| Preoperative medications | Amiodarone, metoprolol tartrate, apixaban, irbesartan, tamsulosin, simvastatin, levothyroxine |
| 5 days pre-op | Apixaban stopped; bridging with enoxaparin initiated |
| Operative day | Robotic epicardial ablation (14 transmural burns) |
| Intraoperative 3D EP mapping using EnSite system | |
| Left atrial appendage ligation with AtriClip | |
| Electrical silence confirmed post-ablation | |
| Post-op Day 5 | Discharged in sinus rhythm; apixaban resumed for 90 days; no antiarrhythmics prescribed |
| 2-week follow-up | Asymptomatic; improved exercise tolerance; resumed daily walking |
| 30-day follow-up | Endocardial catheter ablation (WACA, PWI, mitral isthmus, anterior LA line, CTI ablation) |
| 6-month follow-up | Normal sinus rhythm on ECG; no antiarrhythmic or anticoagulant therapy required |
3 Differential Diagnosis, Investigations, and Treatment
On examination, the patient had an irregular heart rhythm consistent with his known AF. Differential diagnoses included other arrhythmias such as atrial flutter and supraventricular tachycardia. Diagnostic workup included stress echocardiography and 1-month Holter monitoring. During the outpatient evaluation, assessments of frailty were undertaken, including the 5-m walk test and grip strength measurement. The patient's five-meter walk test time was 5.1 s, and his grip strength test result was 12.3 psi. Additionally, key risk scores for patients with AF were calculated. The patient's CHA2DS2-VASc score was 2, indicating a moderate risk of thromboembolism due to AF. His HAS-BLED score was 2, suggesting a moderate risk of major bleeding if anticoagulated. Given these risk scores and the patient's strong motivation to regain his pre-morbid functional capacity, he was deemed an ideal candidate for hybrid ablation to treat his long-standing persistent AF.
3.1 Investigations
3.1.1 Stress Echocardiography
The stress test was performed using the Bruce protocol. The patient achieved stage 1 for 3 min and 1 s, reaching a maximal heart rate of 168 bpm (117% of age-predicted maximum). No chest pain was reported at any point during the testing, though the patient did report a significant exacerbation of his dyspnea. Premature ventricular contractions (PVCs) and AF were noted during stress, and ventricular tachycardia (VT) with frequent PVCs occurred during recovery. The resting left ventricular ejection fraction was 67%, with mild mitral regurgitation at rest. The test was negative for ischemia, and the resting ECG showed nonspecific ST-T wave changes.
3.1.2 Holter Monitoring
One-month Holter monitoring confirmed persistent AF with a variable rate. The baseline rhythm was sustained AF with a heart rate of 60 bpm. There were 0 critical, 7 serious, and 34 stable events recorded, including sustained AF, AF with rapid ventricular response (RVR), atrial flutter with variable conduction, and AF with PVCs. The average heart rate was 74 bpm, with periods up to 150 bpm. No pauses were noted, but clinical correlation was warranted.
3.2 Management
Following the investigations, the patient was evaluated once again in the outpatient clinic, where a decision was made to proceed with the Convergent procedure. This would involve a subxiphoid epicardial ablation with concomitant left atrial appendage (LAA) ligation using the AtriClip ProV system (AtriCure Inc., Mason, OH). Additionally, the decision was made to carry out intraoperative epicardial mapping using the Abbott EnSite 3D mapping system. While epicardial mapping is sometimes undertaken once before starting surgical ablation, we felt that mapping before and after each set of ablations would mean a much more precise set of ablations. It would afford much greater confidence that the patient's AF will be treated, as electrical silence can be confirmed. If any residual aberrant electrical activity is noted, then this can be addressed when the patient returns for the endocardial ablation, and the electrophysiologist will have a specific set of targets.
3.2.1 Operative Course—Epicardial Ablation
The patient's usual anticoagulation (apixaban) was stopped 5 days before the epicardial portion, and the patient was bridged with therapeutic enoxaparin (1 mg/kg twice daily subcutaneously) as is standard at our institution. Preoperatively, a transesophageal echocardiogram showed AF with an ejection fraction of 55% and no left atrial appendage (LAA) thrombus. Three individual 8 mm ports were placed in the left chest wall lateral to the anterior axillary line at the 2nd, 4th, and 6th intercostal spaces, and a 12 mm working port in the sixth intercostal space at the level of the posterior axillary line. A 30° scope was introduced through the 4th intercostal port, and the da Vinci robot was then docked to the patient for robotic left atrial appendage ligation (LAAL). A subxiphoid incision was made, and the pericardium was accessed. The pericardioscopy cannula (AtriCure Inc., Mason, OH) was introduced into the posterior pericardial space, and the coronary sinus, left inferior pulmonary vein, and right inferior pulmonary vein were identified. The EnSite Precision system (Abbott Inc., Chicago, IL) created a 3D electro-anatomical map using esophageal recordings and voltage maps collected by moving the mapping grid over the key epicardial structures.
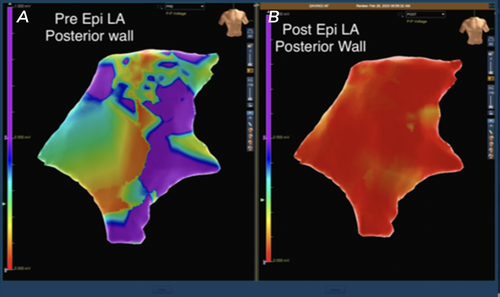
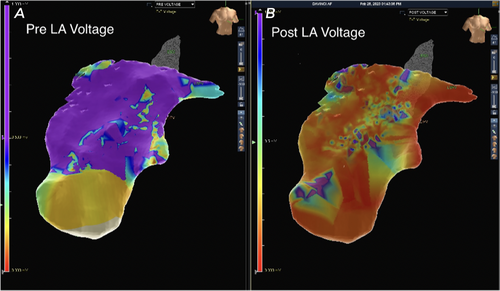
Next, the Epi-Sense ST Ablation Probe (AtriCure Inc., Mason, OH) was introduced through the pericardioscopy cannula. Fourteen transmural burns were made between the left and right inferior pulmonary veins (Figure 1). Increased electrical activity around the right pulmonary vein extending to the dome was detected and ablated. Epicardial mapping confirmed electrical silence, indicating no further aberrant signals or AF circuits. Post-ablation 3D maps showed tissue ablation with a marked reduction in voltage mapping (Figures 2 and 3).
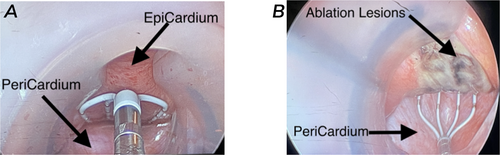
3.2.2 Operative Course—Robotic Left Atrial Appendage Ligation
Attention was then turned to the left side of the chest for robotic LAAL. The phrenic nerve was identified, and the pericardium was entered using a Harmonic scalpel up to the pulmonary artery, maintaining hemostasis throughout. The LAA was visualized, and its size was measured for appropriate clip selection. Under transesophageal echocardiography guidance, the AtriClip device was introduced and deployed around the base of the LAA after confirming proper positioning, with less than 0.5 cm residual stump, as confirmed using at least three angles on TEE. All port sites and the subxiphoid incision were then closed in the standard fashion with two layers of 2/0 Vicryl and 4/0 Monocryl for the skin (both Ethicon Inc., Cincinnati, OH).
4 Outcome and Follow-Up
Postoperatively, the patient's ECG normalized to sinus rhythm without significant events or hemodynamic instability. He was discharged home on postoperative Day 5 on his pre-procedural anticoagulation (apixaban) for 90 days without requiring antiarrhythmic medications. Irbesartan and simvastatin were continued at pre-procedure doses.
At 2-week follow-up, the patient reported feeling well with improved exercise tolerance. He denied chest pain, shortness of breath, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, light-headedness, dizziness, or syncope. He was walking 20–30 min daily, which represented a significantly improved exercise tolerance compared to his preoperative state.
After undergoing the initial epicardial ablation and left atrial appendage clipping, the patient underwent the endocardial portion of the hybrid convergent procedure 30 days later. During this procedure, wide area circumferential ablation, posterior wall isolation, mitral valve isthmus ablation, anterior left atrial line, and caval tricuspid isthmus ablation were performed using a maximum power of 50 W for 30 s anteriorly and 15 s posteriorly, with continuous esophageal cooling to reduce the risk of atrio-esophageal fistula formation.
At 6-month follow-up, the patient was noted to be in normal sinus rhythm on ECG after completing the convergent procedure, with no evidence of sinus dysfunction, atrioventricular block, or bundle branch block. He reported complete resolution of his symptoms with no ongoing requirement for prophylactic anticoagulation or any anti-arrhythmic medication. He will be followed up regularly by his electrophysiologist going forward. The clinical events are summarized in Figure 4.
5 Discussion
In the hybrid Convergent procedure, the surgeon and EP team usually rely on a unipolar or bipolar sensor, which creates several mapping challenges, such as bipolar blindness, that this new 3D cardiac mapping system may be able to overcome. Prior to this technology, the procedure had been performed without intraoperative cardiac mapping. Bipolar blindness occurs when the bipolar sensor accurately maps the activation wavefronts that travel parallel to an electrode pair but is not able to record the wavefronts traveling perpendicular to the sensor [10-12]. This directional sensitivity can cause difficulty in accurately delineating the arrhythmia circuits, which results in more unnecessary ablations or missed circuits [13].
The Omnipolar technology in the 3D cardiac mapping system addresses the challenge of bipolar blindness by providing a 360°, beat-by-beat intraoperative visualization of the voltage activation direction, wave speeds, and max voltage [14]. Using the sensor, the software triangulates the voltage waves and selects the highest amplitude, minimizing directional sensitivity, giving the surgeon a more accurate 3D map of the heart in real-time [11, 12]. This allows the team to pinpoint the exact location of a gap in the ablations, optimizing treatment precision of the Convergent procedure intraoperatively and reducing repeated ablations. Our team avoided unnecessary ablations by verifying that there was no voltage seen on the EGMs post-ablation. The ability to see the signals, or lack thereof, is particularly helpful to avoid unnecessarily ablating the same spot twice. This method also increases procedural efficiency, simultaneously confirming correct catheter positioning. This is especially useful because, while pulmonary vein isolation is usually effective in the treatment of paroxysmal AF, once the disease progresses to persistent AF, more extensive ablation is often performed, increasing the risk of further atrial tachycardias [15].
This case is of a patient with long-standing, persistent AF who underwent the Convergent procedure with the use of real-time 3D epicardial mapping. This real-time electrophysiological mapping of the cardiac tissue after each of the transmural ablations enabled the surgeon to more quickly and precisely determine where tissue needed to be ablated for improved long-term outcomes [14]. While the total procedure time was increased by about 10 min to allow for the 3D mapping, the benefits of more accurate ablation outweigh the small increase in procedure time. The patient tolerated the procedure well, with no postoperative hemodynamic issues.
This patient was an ideal candidate for the Convergent procedure, given his history of long-standing, persistent AF that was refractory to catheter ablations and medical treatments [16-18]. Intraoperatively, the surgeon and EP team were able to more precisely navigate where remaining AF signals were coming from to improve upon subsequent ablations, as well as reduce unnecessary burns to healthy tissues, as seen in Figures 2 and 3. In this case, we could capture information that is often missed or unavailable in traditional mapping systems and apply it during the operations [19]. The patient was discharged home in normal sinus rhythm, which demonstrates an improvement from his preoperative persistent AF.
We believe this case brings to the forefront the novel utilization of intraoperative 3D cardiac mapping with Omnipolar technology that may permit more precise ablations in the treatment of AF. This modality may improve Convergent procedure outcomes by not only reducing unnecessary ablation of healthy tissue but also recognizing persistent electrical signals that may have otherwise been missed with traditional methods [20].
Further studies are needed to determine the long-term effects and benefits of treatment with the use of the intraoperative 3D targeted mapping software compared to more traditional methods. When using conventional mapping, even though there is something that may look ablated, it may still be physiologically active and not silenced [15]. This new mapping technology adds precision. It spares an ablation by looking at high-resolution near and far field waveforms, which allows surgeons to better distinguish ablated tissue, along with seeing the millivolts of the tissue that electrophysiologists would use as criteria for complete ablation [20]. Utilizing this technology minimizes the need for extra ablations, and surgeons can do a more complete and precise job, using the mapping tool as a magnifying glass to really evaluate whether the tissue is a viable substrate for further AF circuits. The limitations of 3D mapping systems include the time required to perform complete mapping and the possibly impaired accuracy of this mapping strategy in more diseased or scarred atria [19]. Furthermore, while our focus was on treating patients with AF in these cases, 3D cardiac mapping holds definite promise for other difficult-to-treat cardiac arrhythmias.
The utilization of real-time intraoperative 3D electrophysiological mapping with EnSite Omnipolar technology significantly enhanced the precision and efficiency of ablations during the hybrid Convergent procedure for persistent AF. This novel mapping approach enabled targeted ablation of affected tissues by providing detailed 3D maps after each transmural ablation, optimizing the overall ablation strategy, and reducing incomplete or unnecessary ablations. The patient was successfully treated and discharged home in sinus rhythm, demonstrating the potential to improve procedural outcomes. The Omnipolar technology addressed bipolar blindness, providing 360° beat-by-beat visualization of voltage activation for more accurate 3D mapping. While modestly increasing procedural time, the benefits of improved precision likely outweigh this.
Author Contributions
Ujjawal Kumar: data curation, formal analysis, investigation, methodology, project administration, visualization, writing – original draft, writing – review and editing. Usman Aslam: data curation, formal analysis, project administration, writing – review and editing. Leslie Epting: data curation, formal analysis, investigation, methodology, project administration, writing – original draft. Anthony Cooper: data curation, investigation, writing – original draft. Alyssa Abraham: project administration, writing – review and editing. Zain Khalpey: conceptualization, data curation, project administration, supervision, writing – review and editing.
Acknowledgments
We would like to thank our patient for permission to publish this case report.
Ethics Statement
This case report was exempt from IRB approval requirements, subject to patient consent.
Consent
Written informed consent was obtained from the patient to publish this report following the journal's patient consent policy.
Conflicts of Interest
L.E. is employed by Abbott Electrophysiology Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be a potential or actual conflicts of interest.
Open Research
Data Availability Statement
The relevant data for this case report are available on reasonable request to Dr Zain Khalpey. The data are not publicly available due to privacy and ethical restrictions.




