Talents Amidst Neurological Impairment; an Interesting Case of Aicardi–Goutières Syndrome
Funding: The authors received no specific funding for this work.
ABSTRACT
Aicardi–Goutières syndrome (AGS) is a rare neuroinflammatory disorder characterized by severe neurological problems and potential overlap with autoimmune disorders. While profound intellectual disability is typically associated with AGS, there have been exceptional cases where individuals exhibit extraordinary talents amidst their neurological impairments. We present a unique and compelling case of a patient diagnosed with AGS who demonstrates remarkable artistic abilities. Despite profound cognitive impairment, the patient exhibits exceptional talent in painting and visualizing objects with extraordinary precision and attention to detail. Unlike most AGS patients, this individual exhibited fewer neurological symptoms and less severe neurodevelopmental impairments. Instead, our patient presented with prominent symptoms of immunodeficiency and exacerbated immune responses. Genetic analysis revealed a specific gene mutation as the underlying cause, contributing to this distinct clinical presentation. Detailed clinical assessments, including neurological evaluations, cognitive testing, and genetic study, were conducted to confirm the diagnosis of AGS and explore the extent of the patient's artistic abilities. This remarkable case of a patient with Aicardi–Goutières syndrome (AGS) challenges our understanding of the cognitive abilities within the syndrome. Despite profound cognitive impairment, the patient exhibits exceptional artistic talents, highlighting the presence of unconventional skills amidst neurological impairments. The coexistence of immunodeficiency and exacerbated immune responses in this case further underscores the heterogeneity of AGS and its potential overlap with autoimmune disorders. By documenting and sharing this unique case, we contribute to a deeper understanding of AGS and inspire further research into the interplay between neurodevelopmental disorders and artistic expression. Recognizing and nurturing unconventional talents in individuals with AGS can inform personalized approaches to management and support, leading to improved quality of life and a broader appreciation of the diverse cognitive profiles within this syndrome. Future studies investigating the genetic basis of AGS are crucial for accurate diagnosis, personalized management, and advancing therapeutic interventions.
Summary
- This case of Aicardi–Goutières syndrome (AGS) illustrates the remarkable potential for artistic expression in patients typically affected by profound cognitive impairments.
- Despite severe neurological challenges, the patient demonstrates exceptional artistic abilities, challenging conventional understandings of cognitive capabilities in AGS.
1 Introduction
Aicardi–Goutières syndrome (AGS) is a rare and complex neuroinflammatory disorder characterized by severe neurological problems, skin manifestations, and potential overlap with autoimmune disorders [1]. Although most individuals with AGS exhibit profound intellectual disability, it is intriguing to encounter exceptional abilities amidst such challenges [2, 3]. In this case report, we present a remarkable and rare instance of AGS, where our patient demonstrates an extraordinary talent in painting and visualizing objects with extraordinary precision and attention to detail (Figure 1). AGS is typically associated with a progressive and debilitating course, leading to significant neurodevelopmental regression and lifelong disabilities [3]. Common clinical features include intermittent fever, skin manifestations such as chilblains and ulcers, muscular abnormalities, and elevated levels of interferon-alpha in the cerebrospinal fluid [1, 4]. However, the presence of exceptional talents within the context of AGS presents a unique opportunity to explore the multifaceted nature of cognitive abilities in this syndrome [3]. This case report aims to shed light on the clinical manifestations of AGS while emphasizing the exceptional artistic talent observed in our patient. By documenting and sharing this remarkable ability, we seek to contribute to a deeper understanding of the cognitive heterogeneity within AGS and inspire further research in this domain. Furthermore, this case underscores the importance of considering AGS in individuals presenting with the described clinical features and highlights the need for comprehensive evaluation, multidisciplinary approaches, and ongoing research to optimize management and support for affected individuals. This report serves as a reminder of the potential for hidden talents and strengths, even in the face of significant neurological challenges, and underscores the importance of embracing the unique abilities of individuals with AGS for a more comprehensive understanding of this rare syndrome.

2 Case Report
2.1 Case History and Examination
The male child was born in 2012 from a consanguineous marriage via caesarean section at term, with a birth weight of 3150 g. His current weight is 25 kg, and his height is 123 cm. At birth, his head circumference was 35 cm, which increased to the 50th percentile during the first 6 months (39.5 cm at 3 months and 40.5 cm at 4 months). The child exhibited delayed developmental milestones, including delays in walking and speech, necessitating assistance and occupational therapy for independent mobility by 1 year of age. Additionally, no family members have demonstrated any inflammatory phenotype, and no positive points in the family history regarding similar conditions. The initial presenting symptom at birth was abnormal eye movements and strabismus, which required surgical intervention on both eyes at 4 years of age. At 3 months old, the patient experienced encephalopathy following treatment with ketotifen, with a possibility of an allergic cough as the underlying cause, resulting in a 20-day period of decreased consciousness. This was accompanied by muscle weakness and hypotonia, further complicating his clinical picture. Around 8 months of age, generalized skin lesions began to appear, initially presenting as vesicular and pustular eruptions predominantly affecting the palms and soles. Over time, these skin lesions progressed to involve the entire body (Figure 2).
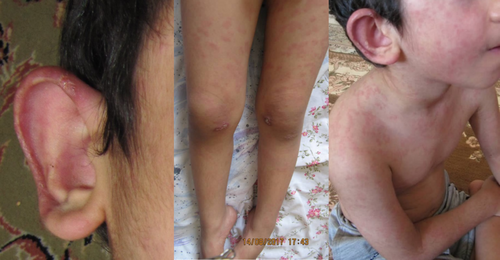
2.2 Treatment, Outcome, and Follow-Up
Multiple consultations with dermatologists eventually led to a diagnosis of erythema multiforme based on biopsies taken from the lesions. As a result, the patient was subsequently referred to a rheumatologist and immunologist for further evaluation. As the patient grew older, recurrent and intermittent fevers with an irregular pattern became prominent. Remarkably, these fevers were not associated with any evident infections or systemic symptoms. The fevers were predominantly nocturnal and coincided with the occurrence of severe skin rashes, initially presenting as erythema and subsequently progressing to ulceration, particularly affecting the ears. Alongside these fever episodes, the patient developed symmetrical joint ulcers in the hands, feet, elbows, knees, and shoulders. Joint warmth, nodules on the hands and feet, and fingertip desquamation associated with erythema were also observed, with symptoms worsening upon exposure to both cold and heat. Fatigue, weakness, and loss of appetite further contributed to the patient's clinical presentation. In this case report, we present the results of liver and kidney enzyme tests and thyroid function evaluation in anormal levels, indicating no abnormalities or dysfunction in these organs. Around the age of 4, when he started kindergarten, his drawing skills became noticeable compared to his peers. As he grew older, this skill gradually improved, revealing an extraordinary talent for drawing and visualizing objects with remarkable precision and attention to detail, despite the absence of any prior history of drawing ability in his family. Additionally, the child's facial appearance, intelligence, and educational development were reported as normal without any apparent concerns. Due to the resemblance of the observed symptoms to periodic fever syndromes, including myalgia, skin rashes, oral ulcers, and growth impairment, investigations were conducted for conditions such as familial Mediterranean fever (FMF), TNF receptor-associated periodic syndrome (TRAPS), and hyperimmunoglobulinemia D syndrome (HIDS). However, these conditions were ruled out through comprehensive evaluations.
Consequently, a comprehensive treatment plan was initiated to manage the systemic inflammatory features. Treatment began at age 6 years with pulse corticosteroids (intravenous methylprednisolone 30 mg/kg/day) administered over 3 days to control acute inflammation and fever episodes. This was followed by oral corticosteroids at a dose of 2 mg/kg/day, which was tapered over 8 weeks based on symptom improvement and tolerance. Concurrently, anakinra (an interleukin-1 receptor antagonist) was introduced at 1 mg/kg/day, subcutaneous, and continued for 3 months to target persistent inflammation, alongside nonsteroidal anti-inflammatory drugs (NSAIDs) administered as needed for joint pain and fever over an initial period of 12 months. This regimen aimed to mitigate the inflammatory cascade, stabilize bone health, and alleviate cutaneous and articular symptoms. Follow-up assessments at 3-month intervals revealed reduced fever frequency and relative improvement in rash severity, though long-term outcomes remain under evaluation. Anakinra was selected as the initial treatment due to its efficacy in targeting interleukin-1 (IL-1), a key mediator in the inflammatory pathways relevant to the patient's symptoms. At the time of treatment, JAK inhibitors were not available in our country. Additionally, we have elaborated on the reasons for the discontinuation of anakinra, emphasizing the significant cost and financial constraints faced by the family, and highlighted the importance of considering this context when evaluating the subsequent clinical outcomes.
Ultimately, genetic testing revealed a homozygous pathogenic mutation in the Aicardi–Goutières syndrome gene. Based on the American College of Medical Genetics and Genomics (ACMG) classification, the c.411_421 variant in the TREX1 gene is pathogenic, conclusively confirming the diagnosis of Aicardi–Goutières Syndrome 1 (Table 1). IFN and ISG expression levels, which could further elucidate the inflammatory mechanisms in AGS1, were not available in our country, representing a limitation of the current study. We emphasize that while these expression levels could provide valuable insights into the underlying pathophysiology, the absence of this data does not detract from the overall findings, including the confirmed TREX1 mutation and the patient's clinical profile. We suggest that future research should aim to include such measurements to further elucidate the relationship between the TREX1 gene mutation and the observed clinical features, potentially enhancing our understanding of AGS1's inflammatory and phenotypic variability.
| Gene | Position/variant | Zygosity | Disease (OMIM) | Inheritance | Classification |
|---|---|---|---|---|---|
| TREX1 |
Chr3:48508492 TCTGGATGGTGC>T NM_007248: exon2 C.411_421del P. Asp138LeufsTer4 |
Homozygous | Aicardi–Goutières syndrome 1, dominant and recessive (225750) | Autosomal recessive/autosomal dominant | Pathogenic |
In the follow-up period after initiating treatment, on low-dose corticosteroid therapy the patient experienced a gradual reduction in fever episodes, which eventually resolved completely. The skin rashes showed relative improvement over time. Joint pain and inflammation were effectively managed and brought under control. He goes to a regular school and gets acceptable grades in his classes, but he needs help with daily tasks and is not completely independent. Approximately 2 years ago, our case was diagnosed with severe osteoporosis, characterized by significantly low bone mineral density (BMD), with a z-score of −3.5 identified in a bone density test. The diagnosis was confirmed through dual-energy X-ray absorptiometry (DEXA) scans, which provided quantitative measurements of BMD. This condition has since been managed with ongoing treatment and regular follow-up care.
3 Discussion
The case we presented involves a pathogenic variant in the TREX1 gene, which is associated with Aicardi–Goutières syndrome 1 (AGS1). Mutations in TREX1 gene are present in approximately 22% of patients [2]. AGS is a rare neuroinflammatory disorder characterized by severe neurological problems, skin lesions, and elevated levels of interferon-alpha in the cerebrospinal fluid [4, 5]. The prevalence of AGS is estimated at more than 4000 worldwide [2]. The neurological damage caused by AGS often leads to profound intellectual disability, spasticity, dystonia, and hypotonia [2]. The clinical features of AGS that we described in our case aligns with these features, such as skin lesions, oral ulcers, muscle abnormalities, spasticity, dystonia and also hypotonia. AGS is known to have a progressive and debilitating nature, with many affected individuals not surviving past childhood [6]. However, it is important to note that some individuals with milder neurological problems or later-onset AGS can live into adulthood [3, 6]. Mortality in childhood ranges from 13% to 35% [6]. Our patient's case deviates from the typical presentation of AGS. Unlike most AGS patients, this individual exhibited fewer neurological symptoms and less severe neurodevelopmental impairments. Instead, our patient presented with prominent symptoms of immunodeficiency and exacerbated immune responses. Genetic analysis revealed a specific gene mutation as the underlying cause, contributing to this distinct clinical presentation. We should mention that some individuals with AGS exhibit features similar to autoimmune disorders, including systemic lupus erythematosus (SLE) [4]. This overlap in symptoms suggests that there may be shared underlying mechanisms between AGS and autoimmune disorders [4, 6]. One specific feature mentioned is the presence of chilblains, which are painful, itchy skin lesions that occur due to inflammation of small blood vessels. Chilblains can be seen in both AGS and SLE [4, 6]. It should be noted that our patient was evaluated for SLE and the results were negative. The encephalopathic phase of AGS causes severe and permanent neurological damage, often resulting in leukodystrophy, which is the loss of white matter in the brain [3]. The presence of calcium deposits (calcifications) in the brain is another characteristic finding in AGS [6]. These neurological abnormalities contribute to the profound intellectual disability, muscle stiffness (spasticity), involuntary muscle contractions (dystonia), and weak muscle tone (hypotonia) observed in individuals with AGS [1, 6]; as in our patient, there were features such as spasticity, dystonia, hypotonia, though no any leukodystrophy and classification due to normal brain MRI. That is indeed a remarkable and interesting observation about our patient. While most individuals with Aicardi–Goutières Syndrome (AGS) experience profound intellectual disability, the manifestation and severity of cognitive abilities can vary significantly among affected individuals [2, 3]. In some cases, individuals with AGS exhibit specific talents or strengths in certain domains despite their broader cognitive impairments [5]. These exceptional abilities, often termed “islands of skills” or “splinter skills,” have been documented in the, literature [5, 7]. Our patient's remarkable artistic talent—characterized by an ability to paint and visualize objects with striking precision and attention to detail—may represent one such example. Although this talent is not a direct consequence of the TREX1 gene mutation, it could be associated with the preservation or enhancement of specific cognitive functions in brain regions unaffected by the mutation's pathology [5]. The medical literature has noted the presence of unique talents in individuals with intellectual disabilities, including those with AGS, though the mechanisms underlying these “splinter skills” remain incompletely understood [5]. We propose that our patient's artistic abilities may reflect the complex interplay between the TREX1 mutation's impact on certain neural circuits and the intact functioning of other brain areas, such as those involved in visual–spatial processing. This highlights the brain's remarkable plasticity and its capacity to support diverse cognitive profiles, even in the context of a severe neurological disorder like AGS [5]. The variability in cognitive outcomes underscores the need for further research into how genetic mutations influence both deficits and strengths. Given this, our patient's talent offers a unique opportunity for clinical and therapeutic exploration. Encouraging these artistic abilities through art therapy or structured engagement in art programs could provide a meaningful outlet for self-expression and communication, potentially enhancing their quality of life. Such interventions may foster personal growth and fulfillment, leveraging a strength that stands out amidst the challenges posed by AGS. Overall, our patient's exceptional capacity for painting and visualizing objects with precision and detail is a rare and noteworthy feature. It not only enriches our understanding of the phenotypic variability in AGS but also emphasizes the potential for preserved brain function to support extraordinary abilities in the presence of a TREX1 mutation. It presents an opportunity for further exploration and support to enhance their artistic skills and overall well-being. During the encephalopathic phase of AGS, affected individuals experience regression in their developmental skills, growth retardation of the brain and skull (microcephaly), and may exhibit intermittent fevers without infection (sterile pyrexias) and seizures [3]. In most cases, patients with Aicardi–Goutières syndrome (AGS) present with severe acquired microcephaly. However, in children with preserved intellect, the head circumference remains within the normal range [3]. In the study conducted by Georgia Ramantani et al., epilepsy was identified as a defining feature of Aicardi–Goutières syndrome (AGS) and was found to typically manifest within the first year of life. The onset of epilepsy is considered a significant milestone in the disease progression and necessitates timely therapeutic intervention [3]. However, it is important to note that in our presented case, the patient did not exhibit any seizures, and was also normocephalic; deviating from the typical pattern observed in AGS. Abnormal findings, such as inflammation-related immune system molecules, can be detected in the cerebrospinal fluid during this phase [8]. These observations support the inflammatory nature of AGS and the impact it has on the central nervous system. In some cases, newborns with AGS may present with features that mimic congenital infections, such as hepatosplenomegaly, elevated liver enzymes, thrombocytopenia, and neurological abnormalities. However, despite the similarities to congenital infection, no actual infection is found in these infants [6, 9]. This highlights the complex nature of AGS and the challenges in diagnosing the condition accurately.
In summary, our presented case demonstrates the key clinical features of Aicardi–Goutières syndrome (AGS), including intermittent fever, skin manifestations such as chilblains and ulcers, and muscular abnormalities. The presence of immunodeficiency and heightened immune responses in our patient, along with milder neurological symptoms, highlights the variability within AGS due to specific gene mutations. Documenting and sharing our patient's exceptional talent can contribute to a better understanding of the cognitive diversity within AGS and stimulate further research. Considering AGS in the differential diagnosis for individuals displaying these clinical features is crucial to ensure appropriate management and support for affected individuals.
4 Conclusion
This case serves as a reminder of the heterogeneity within AGS and the importance of considering this syndrome in the differential diagnosis of individuals presenting with the described clinical features. Accurate diagnosis is crucial for appropriate management and support, including targeted interventions and enhancing overall quality of life. Additionally, documenting our patient's unique artistic talent contributes to understanding the cognitive abilities within AGS. Comprehensive evaluations, ongoing research, and multidisciplinary approaches are vital in managing AGS. Understanding the genetic basis of AGS is crucial for accurate diagnosis and personalized management. Further research is needed to unravel the underlying mechanisms and improve support for individuals with AGS.
Author Contributions
Pooneh Tabibi: conceptualization, data curation, writing – original draft. Reza Shiari: project administration, supervision. Samin Sharafian: resources, supervision, validation. Sara Shiari: data curation, investigation, writing – review and editing.
Acknowledgments
The authors have nothing to report.
Consent
Written informed consent was obtained and signed from the patient's parents regarding the use of the patient's health information for the purpose of writing and publishing a case report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data and materials used in this research are available upon request. Researchers interested in accessing the data and materials may contact [email protected].




