A Case Report of IgA-κ Type Multiple Myeloma Complicated With Hyperlipidemia
Funding: This work was supported by Henan Provincial Traditional Chinese Medicine Culture and Management Research Project (Grant TCM2021004).
Jie Li and Jiashan Zhao have equally contributed to this paper.
ABSTRACT
In the majority of cases, patients with multiple myeloma (MM) generally exhibit normal or decreased blood lipid levels. However, a minority of cases demonstrate the significant role of M protein in elevated lipid levels, particularly in the close association between IgA type MM and hypertriglyceridemia and hypercholesterolemia. Here, we report a case of IgA-κ type MM with concurrent hyperlipidemia, wherein the patient presented with elevated lipid levels upon admission: total cholesterol of 10.44 mmol/L (normal range: 2.8–5.6 mmol/L) and triglycerides of 28.45 mmol/L (normal range: 0.45–1.70 mmol/L). The patient also had concurrent multiple myeloma and was treated with the PCD regimen along with lipid-lowering measures, hydration, alkalization, liver protection, and symptomatic supportive care, and showed a significant improvement in symptoms and was discharged. The aim was to explore the relationship between IgA type MM and hyperlipidemia, and then provide guidance for clinical diagnosis and treatment.
Summary
- IgA-κ type multiple myeloma can cause severe hyperlipidemia due to M protein interference with lipid metabolism, complicating clinical outcomes.
- Early recognition and treatment are critical.
- A comprehensive approach, including the PCD regimen, lipid-lowering therapies, and supportive care, is essential for improving prognosis and managing both the myeloma and metabolic issues.
1 Introduction
Multiple myeloma is characterized by the clonal proliferation of malignant plasma cells in the bone marrow, accompanied by the excessive production of monoclonal immunoglobulins or light chains (M protein) [1, 2]. The malignant proliferation of plasma cells and abnormal levels of immunoglobulins lead to clinical symptoms characterized by CRAB—namely C: hypercalcemia, R: renal impairment, A: anemia, and B: bone pain—, skeletal changes, or pathological fractures [3]. Depending on the type of immunoglobulin secreted by the abnormal myeloma cells, MM can be classified into eight types: IgG, IgA, IgM, IgD, IgE, biclonal, non-secretory, and light chain (κ, λ) [4, 5].
The majority of MM patients exhibit normal or decreased blood lipid levels. However, isolated cases have reported the significant role of M protein in elevated lipid levels [6-8]. Among these cases, IgA type multiple myeloma is particularly associated with hypertriglyceridemia and hypercholesterolemia.
2 Case History/Examination
A 41-year-old male patient presented to our outpatient clinic with a history of fatigue and dizziness for over 6 months and a recent discovery of abnormal blood lipids within the past week. The patient had previously undergone a health checkup a year ago, which revealed elevated triglyceride levels (specifically, triglycerides: 3.65 mmol/L). The symptoms started over 6 months ago following an infection with the novel coronavirus. The patient reported a history of alcohol consumption for more than 10 years, with a daily intake of one or two drinks. Additionally, he had a smoking history of more than 10 years, smoking 4–5 cigarettes per day. There was no family history of genetic disorders.
3 Methods (Differential Diagnosis, Investigations, and Treatment)
Initially, the patient underwent complete blood count, liver and kidney function tests, and coagulation function evaluation. In accordance with the results of blood routine tests, liver and kidney function tests, as well as coagulation function tests, the patient's red blood cell count was 2.73 × 1012/L, and the hematocrit was 27.2%, significantly lower than the normal range. Furthermore, the triglyceride level was 28.45 mg/dL, higher than the normal range. The erythrocyte sedimentation rate (ESR) was measured at 100 mm/h. Preliminary considerations suggest the presence of anemia and hyperlipidemia, as indicated in Table 1.
| Category | Indicator | Results | Reference values |
|---|---|---|---|
| Complete blood count | RBC | 2.73 L × 1012/L | 4.3–5.8 × 1012/L |
| Hct | 27.2 L(%) | 40–50 L(%) | |
| Hematocrit | ALT | 132.0 | 9–50 |
| AST | 44 | 15–40 | |
| Total Cholesterol | 10.44 mg/dL | 2.83–5.17 | |
| Triglycerides | 28.45 | 0–1.7 | |
| HDL | 2.28 | 1.15–1.94 | |
| Coagulation function | aPTT | 54.9 | 21–35 |
| Fibrinogen | 1.34 | 2–4 | |
| PT | 1 | 12–20 | |
| ESR | 100 Hmm/h | 0–15 Hmm/h |
- Abbreviations: ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; ESR, erythrocyte sedimentation rate; Hct, hematocrit; HDL, high-density lipoprotein; PT, prothrombin time; RBC, red blood cells.
To refine the diagnostic findings, the patient underwent gastrointestinal endoscopy, tumor marker examination, thyroid examination, and abdominal MRI. The MRI revealed right renal cyst and abnormal signals in the scanned field of bone, considering the clinical context, suggestive of manifestations related to anemia. Gastrointestinal endoscopy was performed to assess clinical signs in the patient's gastrointestinal tract and detect occult blood in the stool. The results indicated chronic atrophic gastritis, with no significant findings in the rectum or colon. Thyroid ultrasound and functional tests showed no abnormalities. Infection screening also showed no abnormalities. β2-Microglobulin was elevated at 9.3 mg/L. Considering the relevant examinations, the patient's bone abnormalities were consistent with manifestations of anemia, accompanied by an elevated level of β2-microglobulin.
Further perfection through coagulation factor examination, immune-related investigations, anemia assessment, and protein electrophoresis revealed a decrease in Factor VIII levels and coagulation factor deficiency (Table 2). Immunoglobulin A was elevated at 44.52 g/L, complement C was decreased at 30.42 g/L, and erythrocyte sedimentation rate was elevated at 100.0 mm/h. Serum protein electrophoresis showed M protein at 1.22.3 g/L, and serum immunofixation electrophoresis indicated IgA κ type (Figure 1A,B, Tables 3 and 4).
| Project name | Result | Unit | Reference values |
|---|---|---|---|
| FII:C% | 92 | % | 70–120 |
| FV:C% | 33 | % | 70–120 |
| FVII:C% | 238 | % | 55–170 |
| FX:C% | 65 | % | 70–120 |
| FVIII:C% | 4 | % | 70–150 |
| Factor VIII antibody | 0 | BU | 0–0.6 |
| FIX:C% | 51 | % | 60–150 |
| FXI:C% | 50 | % | 70–120 |
| FXII:C% | 75 | % | 60–150 |
| PT | 14.2 | s | 10–16.9 |
| INR | 1.12 | 0.9–1.22 | |
| APTT | 67.9 | s | 20–47.1 |
| Fbg | 1.68 | g/L | 2–4 |
| TT | 17.4 | s | 12.9–22.9 |
- Abbreviations: APTT, activated partial thromboplastin time; Fbg, fibrinogen content; INR, international normalized ratio; PT, prothrombin time; TT, thrombin time.
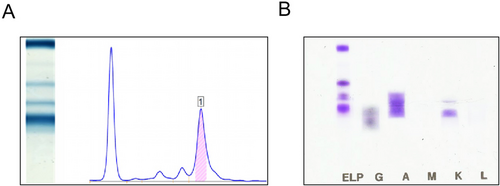
| Ordinal | Codename | Name | Results | Reference values | |
|---|---|---|---|---|---|
| 1 | Albumin | 43.9% | 59.8%–72.4% | ||
| 2 | Alpha1 | α1 | 1.5% | ↑ | 1.0%–3.2% |
| 3 | Alpha2 | α2 | 6.6% | 7.4%–12.6% | |
| 4 | Beta | β | 6.1% | 7.5%–12.9% | |
| 5 | Gamma | γ | 41.9% | ↓ | 8.0%–15.8% |
| 6 | M1 | 22.3 g/L |
| Name | Results | Prompt | Unit | Reference values | Remark | ||
|---|---|---|---|---|---|---|---|
| IgG | Immunoglobulin G | 3.35 | g/L | ↓ | 7–16.0 g/L | ||
| IgA | Immunoglobulin A | 52.981 | g/L | ↑ | 0.7–4 g/L | ||
| IgM | Immunoglobulin M | 1.59 | g/L | 0.4–2.3 g/L | |||
| KAP | Kappa light chain | 17.87 | g/L | ↑ | 2–4.4 g/L | ||
| LAM | Lambda light chain | 1.611 | g/L | 1.1–2.4 g/L |
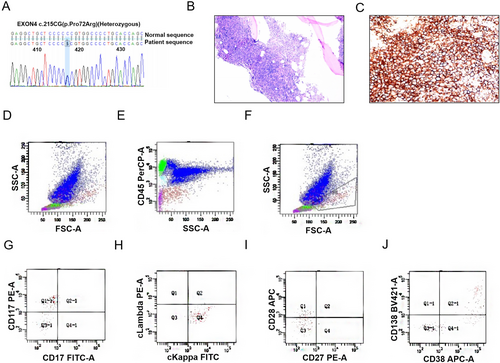
To further enhance the diagnostic workup, bone marrow morphology, bone marrow flow cytometry immunophenotyping, bone marrow biopsy, and bone marrow chromosomal karyotype analysis were performed. Additionally, MRI scans of the cervical, thoracic, and lumbar spine, whole-body skeletal digital radiography (DR), and cardiac ultrasound to rule out amyloidosis were conducted. Following the comprehensive examinations, the findings indicated that the MRI of the cervical, thoracic, and lumbar spine suggested T12 fat deposition, with general signal enhancement in the vertebral bodies, indicating a hematological system disorder. Meanwhile, exon testing revealed a mutation in exon 4 of TP53 (Figure 2A). Bone marrow smears showed mature red blood cells arranged in a coin-like pattern (Figure 2B), with a concurrent presence of 5.5% myeloma cells. Additionally, bone marrow flow cytometry detected abnormal plasma cells (Figure 2D–J) and monoclonal cell proliferation (approximately 60%) (Figure 2C), suggesting plasma cell myeloma (PCM, KAP type).
According to the findings from serum protein electrophoresis, protein immunofixation, bone marrow biopsy, and other relevant examinations, a definitive diagnosis of multiple myeloma was established (high-risk group, DS Stage II, R-ISS Stage III).
Treatment principles were outlined as follows: 1. Due to the patient's young age, targeted therapy for the underlying disease was initiated promptly, aiming for a deep response within four cycles, followed by hematopoietic stem cell transplantation. 2. Given the elevated triglycerides, treatment included fenofibrate, elotuzumab, and plasma exchange therapy. During the treatment process, after plasma exchange, the patient underwent the PCD regimen (bortezomib 1.3 g/dL on Days 1, 4, 8, and 11 plus cyclophosphamide 0.3 g/dL plus dexamethasone 40 mg/dL on Days 1–4, 9–12, 17–20, and 11–12) [9], supplemented with lipid-lowering measures, hydration, alkalization, liver protection, and symptomatic supportive care. Subsequently, there was a noticeable improvement in symptoms, leading to discharge.
4 Discussion
The patient presented with elevated lipid levels upon admission, with a total cholesterol of 10.44 mmol/L (normal range: 2.8–5.6 mmol/L) and triglycerides of 28.45 mmol/L (normal range: 0.45–1.70 mmol/L). Blood routine tests showed a red blood cell count of 2.79 × 1012/L and hemoglobin of 98 g/L. Coagulation profile revealed an APTT of 54.8 s, fibrinogen of 1.23 g/L, and a prothrombin time of 22.6 s. The initial diagnosis included anemia to be further investigated, severe hyperlipidemia, and coagulation dysfunction, with no consideration of multiple myeloma (MM) at first. Subsequently, an elevation in globulins was noticed, and MRI indicated bone changes. Further screening through serum protein electrophoresis revealed the presence of M protein. Following immunofixation electrophoresis, bone marrow biopsy, flow cytometry, and other relevant examinations, the diagnosis was confirmed as multiple myeloma, DS Stage II, and R-ISS Stage III. In this case, the significantly elevated lipid levels, coupled with the patient's frequent social engagements, could easily be mistaken for hyperlipidemia resulting from an unhealthy lifestyle, potentially leading to misdiagnosis. The awareness of abnormal globulin elevation, along with abnormal coagulation mechanisms, emphasized the importance of focused screening for hematological disorders, ultimately leading to the accurate diagnosis of MM.
According to recent research findings, IgA type multiple myeloma (MM) is closely associated with the occurrence of hyperlipidemia. In IgA MM, the M protein in serum protein electrophoresis often appears in the α2 region, and flame-shaped cells are observed in the bone marrow [10, 11]. This type of MM is frequently accompanied by renal impairment, as well as elevated cholesterol and hyperlipidemia. Dyslipidemia, as an independent laboratory indicator, is often linked to lipid metabolism abnormalities, such as hypercholesterolemia, hypertriglyceridemia, and even chylomicronemia syndrome. Clinically, dyslipidemia is often associated with manifestations such as xanthomas, hyperviscosity syndrome, and atherosclerosis [12-14]. Therefore, these findings suggest the importance of closely monitoring the connection between hyperlipidemia and MM in clinical practice. Hyperlipidemia can serve as a significant laboratory indicator, especially for IgA κ type MM.
There is currently no definitive consensus on the mechanism underlying the association between MM and hyperlipidemia [6, 15, 16]. It is widely believed that the monoclonal immunoglobulin produced by malignant proliferation interacts with serum lipoproteins, tissue receptors, and lipoprotein lipase, leading to a reduced clearance rate of lipoproteins. Corsini et al. reported a case of monoclonal gammopathy of undetermined significance (MGUS) containing LDL (low-density lipoprotein) receptor antibodies [17]. Nozaki et al. confirmed this observation, demonstrating that IgA monoclonal immunoglobulin in an MM patient binds to LDL, leading to hyperlipidemia [18]. Kilgore et al. isolated Fab fragments of IgA from MM patients, showing binding activity to LDL, which hindered the LDL receptor pathway, impeding normal lipoprotein metabolism [19]. Cortese compared the metabolism of intermediate-density lipoprotein (IDL) and LDL in two male MM patients with hyperlipidemia [20]. Compared to the control group, MM patients exhibited impaired metabolism of IDL, the process of IDL transitioning to LDL, and the binding capacity of LDL to receptors. These studies suggest that the monoclonal immunoglobulins, especially IgA κ in MM patients, may disrupt the interaction between LDL and its receptors, thereby disrupting lipid metabolism. LDL plays a crucial role in transporting cholesterol from the liver to other organs for utilization, and obstruction of the LDL pathway results in cholesterol accumulation in the bloodstream, leading to hyperlipidemia. In this case, the MM patient presented with an elevated LDL level of 3.54 mmol/L (normal range: 0–3.12 mmol/L), consistent with literature reports. This elevation may be attributed to the disruption of the LDL pathway metabolism by IgA monoclonal immunoglobulins, leading to hyperlipidemia. However, some propose that MM cells rely on exogenous cholesterol for survival, and LDL-cholesterol serves as a crucial anti-apoptotic agent [21]. Hence, it is also plausible that hyperlipidemia induces abnormal lipid metabolism in the body, triggering the production of monoclonal immunoglobulins and eventually developing into MM. Currently, the prevailing theory suggests that MM induces hyperlipidemia, but the causal relationship between the two awaits further investigation [22].
In conclusion, this article reports on a case of IgA κ type MM accompanied by hyperlipidemia. Severe hyperlipidemia may trigger atherosclerosis and cardiovascular diseases, thereby exacerbating the condition of MM and complicating clinical treatment. When patients exhibit hyperlipidemia, clinicians should emphasize the correlation with MM, especially IgA κ type MM, based on laboratory tests and clinical indicators. Early detection and intervention are crucial to address the challenges posed by the coexistence of hyperlipidemia and MM, as severe hyperlipidemia can potentially worsen the prognosis, leading to increased difficulty in clinical management.
5 Conclusions
This case highlights the importance of a comprehensive and timely treatment approach in managing high-risk multiple myeloma complicated by severe hyperlipidemia. The combination of targeted therapy, including the PCD regimen, lipid-lowering strategies, and supportive care, resulted in significant clinical improvement. This underscores the need for individualized treatment plans that address both the underlying disease and associated metabolic complications to optimize patient outcomes and ensure successful management of complex cases like this.
Author Contributions
Jie Li: conceptualization, data curation, formal analysis, investigation, writing – original draft, writing – review and editing. Jiashan Zhao: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, writing – original draft, writing – review and editing. Songyun Wang: investigation, methodology, validation. Rundong Wu: validation, writing – review and editing. Shuyi Duan: validation, writing – review and editing. Huirui Wang: methodology, project administration, validation.
Consent
The authors confirm that the patient has given written informed consent for the publication of this case report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data supporting the findings of this study are publicly available on PubMed and can be accessed using the DOI.




