Successful pembrolizumab treatment of microsatellite instability-high intrahepatic cholangiocarcinoma: A case report
Key Clinical Message
The tumor reduction effect of pembrolizumab is extremely high compared to standard chemotherapy and might show prolonged survival. Therefore, the MSI status should be examined in patients with cholangiocarcinoma.
1 INTRODUCTION
Pembrolizumab is considered to be an effective therapy for patients with microsatellite instability (MSI)-high cancer. Here, we report a case of MSI-high cholangiocarcinoma effectively treated with pembrolizumab. MSI status should be actively evaluated in patients with cholangiocarcinoma.
Cholangiocarcinoma is considered to be intractable and has a poor prognosis. Gemcitabine plus cisplatin is recommended as first-line chemotherapy for patients with advanced cholangiocarcinoma based on an ABC-02 study.1 The median overall survival (OS) after gemcitabine and cisplatin treatment is 11.7 months compared to gemcitabine monotherapy, which is 8.1 months. Furthermore, the median OS of gemcitabine/cisplatin/S-1 (GCS) combination therapy was 16.2 months, compared to gemcitabine and cisplatin therapy, and is becoming one of the standard chemotherapy for advanced cholangiocarcinoma in Japan.2
Recently, immune checkpoint inhibitors (ICIs) have shown promising responses in cancer treatment, regardless of primary site.3 Pembrolizumab, a monoclonal antibody against programmed cell death protein (PD-1), was approved for patients with microsatellite instability (MSI)-high and mismatch repair-deficient (dMMR) cancer. However, only a few cases have had pembrolizumab administered for advanced cholangiocarcinoma.
Here, we present a case of MSI-high intrahepatic cholangiocarcinoma (ICC) in which the tumor was markedly reduced by pembrolizumab treatment.
2 CASE REPORT
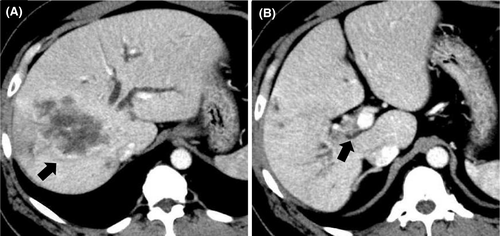
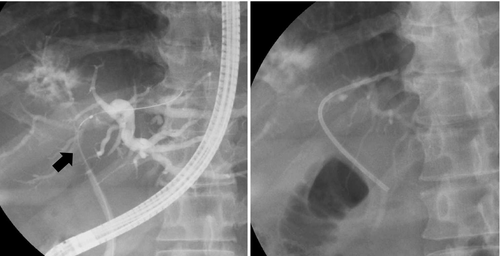
A 50-year-old man presented with epigastric and back pain and was admitted to hospital. Laboratory data revealed alkaline phosphatase (ALP) 824 U/L (106-322 U/L), ɤ-GTP 401 U/L (5-50 U/L) and carcinoembryonic antigen (CEA) 21.1 ng/mL (0-5.0 ng/mL; Table 1). Abdominal computed tomography (CT) revealed a 98-mm tumor in segment 7/8 of the liver with right portal vein tumor thrombosis (PVTT) and dilation of the left intrahepatic bile duct (Figure 1A, 1B). Endoscopic retrograde cholangiopancreatography showed a stricture in the hilar bile duct (Figure 2A). A plastic stent (7 Fr, 12 cm at a deep angle) was placed in the biliary stricture (Figure 2B). Biopsy specimens revealed an adenocarcinoma. However, the patient was diagnosed with an unresectable tumor due to insufficient residual liver volume and was administered GCS combination therapy as outlined in Figure 3. After six cycles, the CEA level was found to be elevated at 23.8 ng/mL, with CT showing an enlarged right PVTT. Oral S-1 combined with radiotherapy (36 Gy/12 fractions) was initiated as second-line therapy for the right PVTT; however, the liver tumor was enlarged to 102 mm. (Figure 4A).
| <Peripheral blood> | <Blood chemistry> | <Tumor markers> | |||
|---|---|---|---|---|---|
| WBC | 8160/μL | TP | 6.4 g/dL | CEA | 21.1 ng/mL |
| RBC | 370 × 104/μL | Alb | 3.0 g/dL | CA19-9 | 2.5 U/mL |
| Hb | 11.7 g/dL | T. Bil | 0.9 mg/dL | ||
| Ht | 35.4% | D. Bil | 0.3 mg/dL | ||
| Plt | 28.2 × 104/μL | AST | 33 U/L | ||
| ALT | 29 U/L | ||||
| <Coagulation> | LDH | 194 U/L | |||
| PT-INR | 0.95 s | ALP | 824 U/L | ||
| APTT | 27.3 mg/dL | γ-GTP | 401 U/L | ||
| FBG | 384 | AMY | 50 U/L | ||
| BUN | 8.8 mg/dL | ||||
| Cr | 0.71 mg/dL | ||||
| Na | 142 mEq/L | ||||
| K | 3.8 mEq/L | ||||
| Cl | 107 mEq/L | ||||
| Ca | 8.8 mg/dL | ||||
| CRP | 4.76 mg/dL | ||||
| FBS | 131 mg/dL | ||||
| HbA1c | 5.0% | ||||
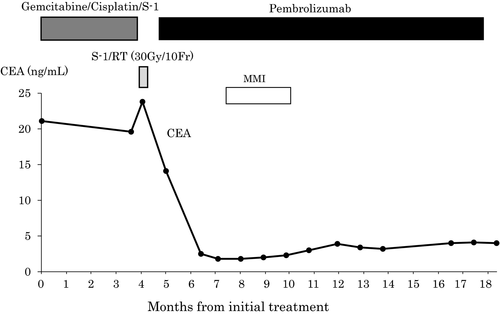
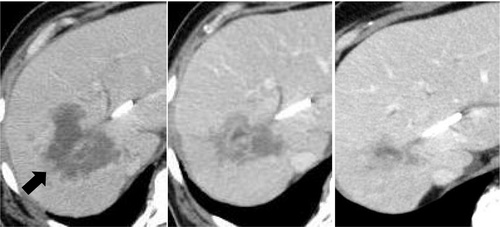
MSI status was investigated using an approved kit (MSI-IVD kit, FALCO biosystems). An analysis of liver tumor biopsy specimens showed a high MSI status; the patient was administered pembrolizumab (200 mg, every 3 weeks). After three cycles of pembrolizumab, the CEA level declined to within normal range (2.3 ng/mL), with CT showing a reduction of 69mm and 32% primary tumor reduction (Figure 4B), leading to a partial response (PR). After 14 cycles, CT revealed a reduction of 21mm and 79% primary tumor reduction (Figure 4C), maintaining the PR; however, the PVTT remained. Four cycles after initiating pembrolizumab, grade 2 hyperthyroidism was observed as an immune-related adverse event. Methimazole was administered for three months, and the patient's thyroid function improved.
3 DISCUSSION
We report a case of advanced cholangiocarcinoma treated with ICI that showed a high response to pembrolizumab monotherapy after standard chemotherapy. Pembrolizumab, is an anti–PD-1 monoclonal antibody that has shown antitumor activity in various types of cancers, including nonsmall-cell lung cancer, gastric cancer, urothelial cancer, and melanoma.4-7 MSI-high/dMMR is considered a potential biomarker of the response to pembrolizumab in several types of cancers.8 Pembrolizumab was approved for previously treated unresectable or metastatic MSI-high/dMMR cancers in Japan. Based on a phase II KEYNOTE-158 study that enrolled 233 patients with MSI-high/dMMR noncolorectal cancer, the objective response rate (ORR) was 34.3% (95% confidence interval [CI], 28.3-40.8%), the median progression-free survival (PFS) was 2.1 months (95% CI, 2.4 to 4.9 months), and OS was 23.5 months (95% CI, 13.5 months to not reached) for MSI-high/dMMR noncolorectal cancer.3
Only three case reports exist of MSI-high advanced cholangiocarcinoma treated with pembrolizumab.9-11 All cases showed a good response and maintained a PR during pembrolizumab treatment. In prior studies of pembrolizumab treatment for advanced cholangiocarcinoma,3 pembrolizumab showed a certain efficacy, with a ORR of 6-17%, median PFS of 1.5-2.1 months, and a median OS of 4.3-7.4 months for a negative or unknown MSI status12-15 (Table 2). However, the KEYNOTE-158 study,3 which included 22 patients with MSI-high cholangiocarcinoma, showed a better ORR of 40.9% (95% CI, 20.7-63.6%), and longer median PFS and OS of 4.2 months (95% CI, 2.1 months to not reached) and 24.3 months (95% CI, 4.1 to 24.9 months), respectively, compared to patients with MSI-negative or unknown. Pembrolizumab in patients with MSI-high cholangiocarcinoma showed a better therapeutic effect than in patients with an unknown MSI status. Compared with gemcitabine plus cisplatin in the ABC-02 study,1 pembrolizumab induced better tumor shrinkage and prolonged survival (24.3 months vs 11.7 months). Thus, pembrolizumab should be considered for first-line therapy for MSI-high advanced cholangiocarcinoma in future.
| References | N | MSI, % | ORR(%) | PFS(M) | OS(M) |
|---|---|---|---|---|---|
| Bang et al13 | 24 | NA | 17 | NA | NA |
| Lee et al14 | 51 | NA | 10 | 2.1 | 6.9 |
| Kang et al15 | 40 | NA | 10 | 1.5 | 4.3 |
| Piha-Paul et al16 | 104 | Negative, 95 | 6 | 2 | 7.4 |
| Missing, 5 | |||||
| Marabelle et al4 | 22 | High, 100 | 41 | 4.2 | 24.3 |
Note
- Abbreviations: NA, not available; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
In our case, pembrolizumab monotherapy for ICC refractory to GCS combination therapy was found to be effective, with a PR maintained 13 months after treatment. The frequency of MSI-high in all cholangiocarcinomas is only 2%,16 and the rate for benefiting from pembrolizumab is low. However, since patients with MSI-high cholangiocarcinoma administered pembrolizumab might show prolonged survival compared to standard chemotherapy, it is necessary to examine the MSI status in order to identify patients that might benefit from pembrolizumab treatment.
4 CONCLUSION
MSI-high cases are rare among patients with advanced cholangiocarcinoma; however, the potential therapeutic effect of pembrolizumab is extremely high. Therefore, the MSI status of advanced cholangiocarcinoma cases should be actively examined since some patients can benefit from pembrolizumab treatment.
AUTHOR CONTRIBUTIONS
YI and MO: collected data and wrote and edited the manuscript. GO, SA, MY, TA, and SF: involved in the patient's care. MF: Pathologist who diagnosed this disease. MM: supervised this study.
ACKNOWLEDGMENTS
We thank all editors and reviewers for the comments, which helped us to improve our manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
YI and MO: collected data and wrote and edited the manuscript. GO, SA, MY, TA, and SF: involved in the patient's care. MF: Pathologist who diagnosed this disease. MM: supervised this study.
Open Research
DATA AVAILABILITY STATEMENT
Data available within the article.