Active fixation coronary sinus lead in a patient with persistence of left superior vena cava
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abstract
In patients with PLSVC, the use of an active fixation lead (like the Medtronic “Attain Stability”) on the coronary sinus can lead to a successful and safe cardiac resynchronization, facilitating its positioning with a low long-term displacement rate.
1 INTRODUCTION
Persistent left superior vena cava (PLSVC) is the most common congenital anomaly of the systemic venous return (an overall reported prevalence of up to 0.5% in the total population and up to 10%-20% in patients with congenital heart defects),1 and a device implantation can be challenging, especially in those involving cardiac resynchronization therapy and defibrillator (CRT-D), as the coronary sinus in these patients is almost always very dilated, with small tributary branches; on this basis, it follows that it is very difficult to obtain an acceptable lead stability and thus to achieve optimal cardiac resynchronization. Even though similar cases are present in the literature, no standard implant technique has been identified for patients with this anomaly.
2 CASE REPORT
2.1 History of presentation
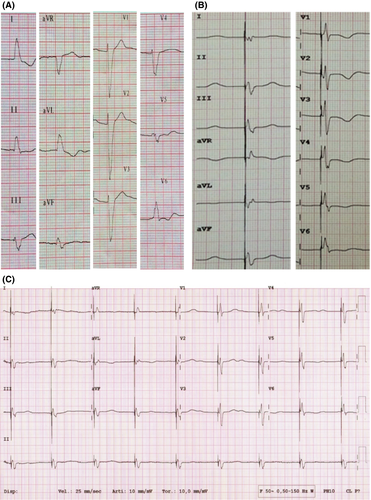
We report a case of a 71-year-old male patient with dilated valvular cardiomyopathy, dyspnea NYHA class III, 30% left ventricular ejection fraction (LVEF), and a 12-lead ECG with sinus rhythm conducted with left bundle branch block (Figure 1A). He had already been treated with heart failure therapy comprising beta-blockers, ACE inhibitors, and diuretics.
His medical history was notable for diabetes, chronic kidney injury, peripheral vasculopathy, prostate cancer, thalassemia minor, and some episodes of acute heart failure.
In 2003, he underwent cardiac surgery (Bentall procedure and mitral valve repair) for rheumatic valvular disease; in 2014, he had catheter ablation of typical atrioventricular nodal re-entrant tachycardia and of typical atrial flutter.
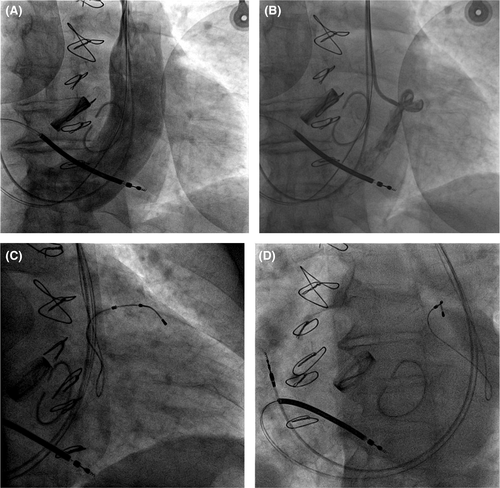
According to the last ESC Guidelines,2 considering the symptoms despite adequate medical treatment, the 12-lead ECG, and the echocardiographic data, CRT-D was indicated. During the implant procedure, intraoperative venography revealed persistent left superior vena cava (PLSVC) and a dilated coronary sinus (CS), with right superior vena cava draining only into the PLSVC via an anastomotic branch (Figure 2A).
2.2 Management
First, a Medtronic Viva XT CRT-D with Medtronic-6935 lead for the right ventricle and a Medtronic-5076 lead for the right atrium were correctly positioned. However, the attempt to position the standard coronary sinus lead was unsuccessful due to the enormous venous flow of the dilated CS and due to the small and narrow branches.
After 2 years, the patient was hospitalized for a further episode of acute heart failure; in that occasion, it was decided to optimize the implantation, attempting with another type of lead (Medtronic Stability 4796-88 active fixation lead). The procedure was performed on a fasting patient, during antibiotic prophylaxis with intravenous cefazolin and under local anesthesia with subcutaneous lidocaine. Initially, percutaneous puncture of the left subclavian vein was performed with the introduction of a wire; an incision was also made on the skin in the left subclavicular region in correspondence with the previous surgical scar. By blunt dissection, the pocket containing the generator was identified and then opened. The left subclavian vein was cannulated, and a subselector was inserted through the left superior vena cava; venography of the coronary sinus was then performed, showing its marked dilation and flow, and only one visible tributary vein (Figure 2B). Then, through a 0.014-inch wire, the bipolar lead with active fixation (Medtronic Stability 4796-88) was placed in the anterolateral vein. After positioning it in the target vessel and screwing the lead, the traction tests revealed good stability and electrical parameters: R wave sensing 11.7 mV, impedance of 832 Ohm, and threshold of 2.2 V at 0.5 ms; the generator was connected to the leads and placed in the previous prefascial pocket. After a final fluoroscopic check, the leads and the device were fixed to the prepectoral muscle band by sutures, and the incision was closed in layers with reabsorbable sutures at detached points of the subcutaneous tissue and with nonabsorbable polymer at detached points of the skin. Device upgrade was performed without complications, with a running time of 80 minutes and a fluoroscopy time of 38 minutes. The final 12-lead ECG showed a narrow QRS, the predischarge chest X-ray showed good permanent position of the leads, and the patient was discharged the following day (Figures 1, 2-D).
2.3 Follow-up
Since the implantation, there have been no recurrences of acute heart failure; the patient has always been asymptomatic, and during the follow-up after 1, 3, and 6 months and 2 years, stable electrical parameters and long-term persistence of a narrow QRS wave on surface 12-lead ECG were observed. A mild improvement in LVEF (35%) and in dyspnea symptoms (NYHA II) was also reported (Figure 1C-3).

3 DISCUSSION
The persistence of the left superior vena cava (LSVC) is the most common asymptomatic congenital alteration of the systemic thoracic venous return.1 LSVC is often connected to the CS (92%), less to the left atrium (LA, 8%); drainage of venous blood from the upper left part of the body and from the left arm is guaranteed via the jugular vein, LSVC, and CS to the right atrium.
Typically, the presence of the upper left vena cava is an asymptomatic and random finding in imaging tests (CT, MRI, or angiography) or during interventions (catheterization, pacemaker or defibrillator implant, thoracic surgery). A dilated coronary sinus detected with echocardiography could suggest the existence of LSVC, which is confirmed after the injection of echocardiographic contrast in the cubital vein. Even an abnormal transvenous passage of guides and leads could suggest the presence of LSVC, showing a characteristic left run before the coronary sinus connection. The persistence of LSVC is associated with the presence of arrhythmias from an accessory or intranodal pathway; therefore, invasive procedures could report severe complications during the positioning of central venous access.3 Due to the low incidence of LSVC in healthy subjects, routine cardiac imaging is recommended only in patients with congenital disease before the implantation of a biventricular device.4
Persistent LSVC is not a contraindication to the subclavian vein catheterization; moreover, imaging could help the operator in choosing the most appropriate approach for the implant (guides, catheters, and stylets) in order to increase the possibilities of success.5, 6
The operator should be aware of anatomical abnormalities, difficulties in electrochemical fixation, and potential procedural complications; in case of LSVC without SVC, it is more difficult to insert the lead in the CS.7
It is important to perform a venous angiography to identify LVSC, to evaluate the angle of the CS orifice and the possible presence of a double vena cava.8 To insert the lead into the CS, it may be useful to use guides with atypical curves.9
Many patients have bilateral SVCs, and the right SVC is a viable option in case of difficulty in the left approach.10 In the absence of right SVC (as in this case), the left subclavian vein remains the only solution.
To the best of our knowledge, there are only a few case reports in the literature regarding CRT-P or CRT-D implantation via PLSVC; most of these are old, and although they describe the use of an active fixation lead in the right atrium and/or ventricle, none of them reports the use of this type of lead in the coronary sinus.11-17
As a result, currently there is no standard implant technique, and often, technical improvisations are required to obtain satisfactory results.
The Medtronic Model 20066/4796-88 LV lead (“Attain Stability,” Medtronic Inc) is a bipolar 88-cm-long lead designed for pacing of the left ventricle with electrodes made of platinum-iridium alloy with titanium nitride coating. The diameter of the lead body and of the electrodes is 1.47 mm (4.4 French) and 1.70 mm (5.1 French), respectively.
It is designed with active fixation (unique side helix mechanism, positioned 0.25 mm away from the lead body before the proximal electrode) to allow for precise placement and secure fixation during implant. This design provides more flexibility in pacing location, reduces the risk of lead dislodgement, and integrates a mechanical stop and adhesive backfill for prevention of over-rotation and vein tissue pinching. With these characteristics, this lead has the potential for use in all types of coronary venous anatomy, especially in those with huge flow toward the coronary sinus as in patients with PLSVC.
4 CONCLUSIONS
In patients with PLSVC, the use of an active fixation lead (like the Medtronic “Attain Stability”) on the coronary sinus can lead to a successful and safe cardiac resynchronization, facilitating its positioning with a low long-term displacement rate.
We know well that our choice is not generalizable because of the variability of the CS anatomy and its tributary veins, the possible presence of a right SVC that makes the right approach possible, and the possibility of surgical implantation of an epicardial LV lead in selected patients; despite this, we think our experience deserves to be shared in literature, being useful in all those cases where these other solutions are not possible.
CONFLICT OF INTEREST
Prof. R. De Ponti has received lecture fees from Biosense Webster and Biotronik, and his institution has received educational grant from Medtronic, Biotronik, Boston Scientific, Biosense Webster, and Abbott.
AUTHOR CONTRIBUTIONS
FC: conceived of the presented idea, contributed to treatment applications, data collection, intellectual process, and manuscript writing, and reviewed and contributed to the final version of the manuscript. MG: contributed to data collection, intellectual process, follow-up, and manuscript writing, and reviewed and contributed to the final version of the manuscript. GD: contributed to data collection, intellectual process, follow-up, and manuscript writing. GO: contributed to data collection, intellectual process, and manuscript writing. ALV: contributed to data collection, intellectual process, and manuscript writing. CG: contributed to data collection, intellectual process, and manuscript writing. EMP: contributed to data collection, intellectual process, and manuscript writing. Prof. RDP: supervised the project. All authors were involved in delivering the key clinical message and contributing intellectually to this case report.