Suppression of postsurgical recurrence of hepatocellular carcinoma treated with autologous formalin-fixed tumor vaccine, with special reference to glypican-3
Key Clinical Message
Autologous formalin-fixed tumor vaccine (AFTV) suppressed re-recurrence for more than 32 months of multiple-recurrent hepatocellular carcinoma based on hepatitis C virus-induced liver cirrhosis in a case with previous recurrence interval, 51-, 28-, 12-, and 4-months. We detected glypican-3-specific cytotoxic T lymphocytes in the peripheral blood at 12 months after AFTV.
Introduction
In patients with chronic hepatitis and in those with liver cirrhosis, detection of increasing incidences of hepatocellular carcinoma (HCC) requires careful monitoring every 3–6 months using ultrasound sonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and frequent blood tests. Once HCC is detected, patients experience repeated recurrence, despite the fact that a variety of HCC treatment methods have been developed, such as anti-viral agents, surgical treatment, percutaneous ethanol injection (PEIT), radiofrequency ablation (RFA), trans-catheter arterial chemoembolization (TACE), and molecular-target drugs.
In the last 10 years, tumor vaccines generated from patients' own formalin-fixed HCC tissues have been developed which can decrease HCC recurrence. The autologous formalin-fixed tumor vaccine (AFTV) has been reported to reduce the recurrence risk of primary resected hepatitis B virus-background HCC by 81% 1. In one case, recurrence of multiple-recurrent hepatitis C virus (HCV)-back ground HCC was clearly suppressed long-term without any additional treatment 2.
Here, we report a case in which AFTV injection resulted in suppression of multiple-recurrent HCV-background HCC recurrence. In this case, we detected a significant density of glypican-3 (GPC3)-specific cytotoxic T lymphocytes (CTL) in the blood at 12 months after the last intradermal injection of AFTV.
Case Report
A 55-year-old female patient was diagnosed with HCV-related hepatitis in 2002. Liver biopsy showed active chronic hepatitis accompanied by bridging fibrosis and necrotic inflammatory reactions. To reduce the activity of chronic hepatitis, the patient was treated with consensus interferon for 24 weeks. Results showed complete remission of the hepatitis as liver transaminases normalized, as well as conversion of seropositive HCV to negative up to the present.
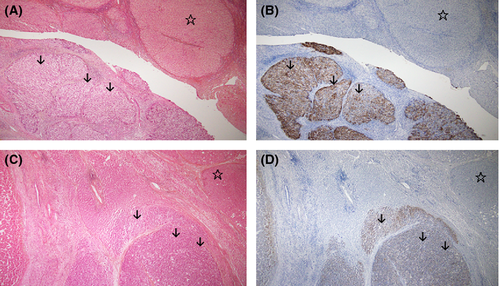
When she was 57 years old, two 20-mm HCCs were observed in left lobes S2 and S3, and a left hepatic lobectomy was performed in June 2004. In addition, two 10-mm HCCs in S5 and S7 were treated with radiofrequency ablation (RFA) in September 2008. The 10-mm HCC in S5 was detected again in January 2011, and was treated with trans-catheter arterial chemoembolization (TACE) and RFA. In January 2012, a new 10-mm HCC was found in S6, and a resection was done after treatment with RFA. Pathologies obtained after the two hepatectomies showed poorly differentiated HCC in the 2004 specimen and moderately differentiated HCC in the 2012 specimen (Fig. 1). The liver function has been kept good and the bilirubin levels were <2 mg/dL. Portal vein pressure was maintained within Child-Pugh A class. The patient did not hope liver transplantation but desired AFTV therapy because of the rapid decline in the recurrence interval, that is, 51-, 28-, 12-, and 4-months.
We prepared AFTV as reported previously 3 using a total of 1.56 g of autologous HCC tissue recovered from formalin-fixed and paraffin-embedded tumors from the two operations. According to the therapeutic schedule employed 1, a single course of AFTV therapy applied from September to October 2012 consisted of the following: (1) A delayed-type hypersensitivity (DTH) test with immunoadjuvant-free fixed HCC fragments applied to a forearm was initiated 48 h before the first AFTV injection to check for allergic reaction. The first DTH response was negative. (2) Up to three rounds of AFTV were injected intradermally into the upper arm at 2 week intervals. (3) A second DTH test, performed 2 weeks after the last AFTV injection to the other forearm, showed a positive DTH response. (4) Ultrasound sonography and blood tests for tumor markers which were conducted every 3 months, and computerized tomography every 6 months, revealed no evidence of recurrence or generation of new HCC lesions for more than 27 months.
Immunohistochemistry to detect GPC3 4 in the HCC specimen resected in 2004 revealed strong expression of GPC in the HCC lesion (Fig. 1B). We also detected GPC3 expression in the 2012 specimen resected after treatment with RFA (Fig. 1D). However, we did not observe any GPC3 expression within cirrhotic lesions in either specimen (Fig. 1B and D).
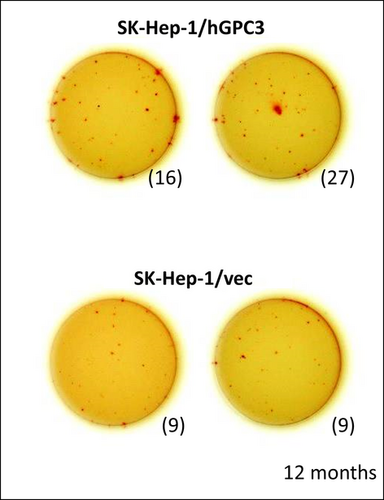
To evaluate AFTV-induced GPC3-reactive CTL, we carried out interferon-γ-specific ELISPOT assays as described previously 5, using a target human HCC cell line expressing GPC3 (SK-Hep-1/GPC3). The cell line was developed from SK-Hep-1/HLA-A0201/2402 cells by transfecting with human GPC3-gene. A control cell line (SK-Hep-1/vec) transfected with the empty vector were also used for detection of GPC3-reactive CTL. Although HLA subtypes of the patient and the target cell line differ (HLA-A0206/3303 and HLA-A0201/2402, respectively), HLA-A2 (A0201)-restricted GPC3 epitopes are cross-reactive with HLA-A0206, allowing detection of GPC3-specific CTL that are reactive to the target cell line 5.
Glypican-3-specific CTLs were clearly observed in peripheral blood drawn 12 months after the first injection of AFTV (average 21.5 spots against SK-Hep-1/hGPC3 cells vs. nine spots against SK-Hep-1/vec control cells) (Fig. 2).
Discussion
GPC3, a heparan-sulfate proteoglycans linked to the outer surface of cell membrane via a glycosylphosphatidylinositol anchor 6, is frequently overexpressed in HCC 7, 8 and in precancerous lesions in the liver 9. For this reason, a recombinant humanized monoclonal antibody against GPC3 10 and a GPC3-peptide vaccine 11 have been developed for treatment of HCC. Treatments with these new agents were well tolerated, and noted measurable immune responses and antitumor efficacy. However, clinical outcome were not necessarily prominent in these Phase I trials compared to the previous expectations derived from preclinical studies.
Autologous formalin-fixed tumor vaccine differs from these specified molecule-targeted agents in that the autologous tumor, containing a wide array of unidentified antigens, is the source of antigens. Thus, CTL induced with AFTV made from tumor tissue must be polyclonal by nature; for example, polyclonal CTL induced in vitro with a formalin-fixed carcinoembryonic antigen protein 12. At present, no one provided any evidence that the impure tumor antigenic formalin-fixed autologous HCC tissue is truly active to induce a specific cellular immune response against a specified tumor antigenic molecule. The present case is the first one we could identify GPC3-specific cellular immune response based on the autologous formalin-fixed HCC.
While we detected GPC3-specific CTL in the patient's blood 12 months after AFTV treatment, few GPC3-specific CTL were detected 16 months after the treatment (Data not shown). These results may suggest that, although the conspicuous life span of GPC3-specific CTL in blood is <16 months, the immunoprotective effect of AFTV continues beyond GPC3-specific CTL lifespan in blood, since this patient presently continued to show no evidence of HCC recurrence 32 months after the second resection of HCC.
We consider two hypotheses to explain this continued protection: (1) The GPC3-specific CTL may have differentiated into memory T cells, which migrate to lymphatic tissues and are therefore not conspicuously detectable in peripheral blood. (2) The GPC3-specific CTL apoptosed after killing all target HCC cells in vivo, thereby eliminating all tumor antigens and preventing replicative stimulation to active CTL.
Conclusion
This is the first case we could identify GPC3-specific cellular immune response based on impure tumor antigenic formalin-fixed autologous HCC tissue. Present results suggest that AFTV should be taken in consideration as one of treatment modalities in cases involving potential HCC derived from chronic hepatitis and liver cirrhosis.
Conflict of Interest
None declared.




