Topiramate-induced nephrolithiasis
Key Clinical Message
Nephrolithiasis is a less common side effect of the antiepileptic drug topiramate. We report the case of a 3-year-old boy who presented to the emergency department with abdominal pain; examinations revealed a large calcification in the left kidney. Regular ultrasound examinations are recommended in children using topiramate.
Case
A 3-year-old boy presented to our emergency department with abdominal pain. His medical history was relevant for profound developmental delay secondary to brain malformation including polymicrogyria and complex-focal seizures. The patient was on topiramate therapy for 1.5 years (dose: 6.5 mg/kg/day; recommended daily dose: 3–9 mg/kg/day).
Ultrasonography and plain abdominal X-ray revealed a large calcification in the left kidney (Fig. 1) with urinary obstruction requiring the insertion of a DJ-catheter. Open surgery with fragmentation and removal of the stone was performed (Fig. 2). Stone analysis of the calculus demonstrated calcium phosphate. Four additional extracorporeal shockwave therapies were performed over time in order to remove all residual stone fragments. After stopping antiepileptic treatment with topiramate and starting levetiracetam treatment, no recurrence of nephrolithiasis was noted. The patient had no family history for nephrolithiasis.
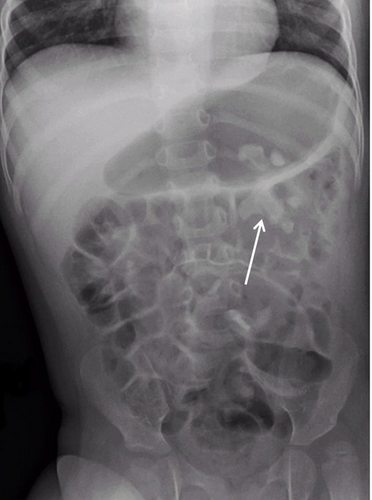

Topiramate is a carbonic anhydrase inhibitor commonly used in patients with focal epilepsy. Chronic use can lead to metabolic acidosis, hypocitraturia, hypercalciuria, and elevated urine pH, factors which increase the risk of calcium oxalate and calcium phosphate stone formation 1. For early detection of renal complications, regular ultrasound examinations of the urinary system and special recommendation for fluid intake is needed during the use of topiramate in children 2.
Conflict of Interest
None declared.




