Deferasirox in a refractory anemia after other treatment options: case report and literature review
Key Clinical Message
Deferasirox, represents an effective iron chelator drug in lower risk myelodysplastic syndromes. Reduction in oxidative stress is responsible of the hematologic improvement but further explanation may impact on its benefit. Biological and clinical studies are necessary to better define mechanisms of action, assess toxicities, and predicting factors of response.
Introduction
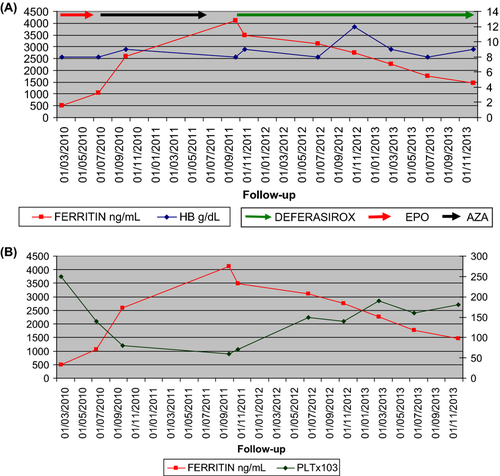
After 4 months of EPO trial (40,000 UI/week) and transfusion dependence (four red cell transfusions per month), Azacytidine was introduced at dose of 75 mg/m2/day subcutaneously 5 days monthly per 11 courses. [Correction added on 8 May 2015, after first online publication: ‘76’ corrected to ‘75’.] No erythroid response was noted. In September 2011 he started Deferasirox 20 mg/kg/day. Hematological restaging confirmed low-risk IPSS myelodysplastic syndrome (MDS). After 12 months of iron chelation therapy (ICT), hematological improvement (HI) was reached (WBC 3600 × 109/L, Hb 11.8 gr/dL PLT 141,000 × 109/L) and ferritin level decreased (2756 ng/mL). After HI, transfusion discontinuation lasted 6 months (from November 2012 to March 2013). In this experience, such as in literature, serum ferritin levels decreased progressively over time and deferasirox was well tolerated with manageable side effects. After 18 months severe anemia relapsed and he required four transfusion per month. Platelets count remained normal and ferritin level continued to decrease (1758 ng/mL). In December 2013 he presented the following parameters: WBC 3170 × 109/L, Hb 9.2 gr/dL, PLT 185,000 × 109/L, ferritin 1469 ng/mL.
Myelodysplastic syndromes (MDS) are hematopoietic stem cell disorders characterized by ineffective hematopoiesis, peripheral cytopenias and risk of progression to acute myeloid leukemia. MDS occur in older patients that suffer from comorbidities 1.
Several prognostic scores of disease are based on anemia and transfusion dependence 2-5. The international Prognostic Scoring System (IPSS), commonly used to predict newly diagnosed MDS, is based on cytopenias, cytogenetic feature, and marrow blasts 2. The World Health Organization (WHO)-based Prognostic Scoring System, used at any time during the course of disease, is based on WHO categories, hemoglobin level and cytogenetic features 3. A new revised International score (IPSS-R) was validated in 2012. This score includes different cutoff for cytopenias ad new cytogenetic categories 4. More recently MD Anderson MDS Lower Risk Prognostic Model (LR-PSS) based on anemia and thrombocytopenia, unfavorable cytogenetics and marrow blasts, is validated to predict the prognosis of “poor prognosis” lower risk disease 5.
Case Report
The patient (male) was diagnosed in March 2010, at the age of 60 with Refractory Anemia (RA) low-risk IPSS and low-risk WPSS: severe anemia, normal karyotype, bone marrow blasts inferior to five percent. Biopsy confirmed the diagnosis according to the WHO criteria. Laboratory features were increased ferritin levels (500 ng/mL), normal vitamin B12 and folate stores, serum erythropoietin (EPO) level of 110 U/L. No mutations of familiar hemocromatosis gene (HFE) were recognized.
The initial therapeutic approach for treatment of the symptomatic anemia was erythropoietin 40,000 UI/week and supportive care. After 4 months of EPO trial and transfusion dependence (four red cell transfusions per month) treatment was stopped 6. In August 2010, 5-azacytidine was introduced at dose of 75 mg/m2/day subcutaneously 5 days monthly. The side effect profile of the drug was manageable, the treatment was performed for 11 cycles, but no erythroid response was noted 7. As a consequence of ineffective erythropoiesis and blood transfusions, ferritin levels increased.
In September 2011, he started deferasirox (ICL670) 20 mg/kg/day. Hematological restaging confirmed RA low-risk WPSS, ferritin level further increased (4124 ng/mL). The blood cell count was: White blood cell count (WBC) 2040 × 109/L, hemoglobin (Hb) 7.9 gr/dL, platelet count (PLT) 64 × 109/L.
After 12 months of iron chelation therapy (ICT), hematological improvement (HI) was documented (WBC 3600 × 109/L, Hb 11.8 gr/dL PLT 141,000 × 109/L) and ferritin level decreased.
Transfusion discontinuation lasted 6 months, from November 2012 to March 2013. In this experience, such as in literature, transfusion independence was obtained and maintained, reduction in ferritin levels was anticipated by a decreased transfusion requirement 8-17.
In addition, deferasirox was well tolerated. During the first month of therapy temporary discontinuation due to increased serum transaminases was necessary. Neither gastrointestinal disturbance nor increased serum creatinine were observed. Three year of follow-up of ICT did not show any auditory or ocular adverse events.
After 18 months of deferasirox, severe anemia relapsed and transfusion dependence reappeared (four transfusions per month). Platelets count remained normal and ferritin levels continue to decrease (1758 ng/mL). In December 2013, he presented the following parameters: WBC 3170 × 109/L, Hb 9.2 gr/dL, PLT 185,000 × 109/L, ferritin 1469 ng/mL.
Hematologic restaging confirmed the diagnosis of RA according to WHO criteria, low-risk WPSS. History and physical examination such as laboratory features did not show any significant further data (bleeding, vitamin deficiency, hemolytic syndrome, infections or other chronic disorders).
This case may suggest a possible positive effect of the drug on hematopoiesis of a low-risk MDS. Major erythroid and platelet responses were recognized according to the International Working Group (IWG) 2006 criteria 8.
A possible hypothesis of the erythroid relapse may be genomic instability of MDS itself plus reactive oxygen species due to iron excess consequence of ineffective erythropoiesis and blood transfusions. Another possibility may be the occurrence of additive “noxa” effect on the hemopoietic tissue or stromal cells (Fig. 1).
Interestingly, ICT was continued until the last follow-up, November 2014, with good compliance and stable hematologic parameters: hemoglobin, platelet, and ferritin level. Furthermore, transfusion dependence (four transfusions per month) remains stable. Obviously, clinical and laboratory follow-up and accurate restaging are mandatory.
Results and Discussion
Treatment of MDS varies according to risk group. Lower serum EPO (<500 mU/mL) and a lower pretreatment red blood transfusion (RBC) requirement (<2 units per months) were commonly associated with a higher EPO response rate 6. AZA is generally administered at a dose of 75 mg/m2/day subcutaneously for 7 day in patients with progressing or high-risk disease.
In this patient, category 2 according to LR-PSS (anemia, thrombocytopenia, and age) an alternative 5-day schedule of AZA was used 7. Subsequently, deferasirox was introduced after AZA failure.
It seems rationale, as reviewers and guidelines suggest, an earlier introduction of ICT in transfusion-dependent MDS (20–25 RBC units, serum ferritin >1000 or >2000 ng/mL) in order to reduce the body iron burden and further toxicities.
Many authors reported the hematological improvement after deferasirox treatment in MDS. It is of interest to determine factors associated with hematologic response and mechanisms of relapse. Reduction in oxidative stress has been proposed as main explanation for this improvement and alternative mechanisms may include redistribution of iron, effect on bone marrow microenvironments or on the neoplastic clone 8-17.
RBC transfusion dependence is observed in higher risk MDS but also contributes to iron overload in lower risk disease due to increased absorption of iron from the gut. Hecpidin, a key negative regulator of iron export from gut and macrophages, is typically suppressed in conditions such as thalassemia or ineffective erythropoiesis 18. More recently, Santini et al. found different levels of hepcidin in different types of myelodysplastic syndromes. Lower level of the protein was demonstrated in low-risk disease 19.
Humans lack a physiological excretion mechanism for excess iron and each RBC unit contains approximately 200–250 mg of elemental iron 18. In addition, excess iron has been shown to suppress erythropoiesis, as evidenced by decreased burst-forming units erythroid (BFU-E) in patients with elevated ferritin 20.
Transfusional iron causes accumulation of labile plasma iron (LPI). This pathological form of nontransferrin-bound iron (NTBI) that take up by cells, lead to a rise in labile iron pool (LIP) and generate reactive oxygen species (ROS). It is well known that oxidative stress leads to oxidation of proteins, lipids, and DNA with increased apoptosis, organ damage, and genomic instability 21, 13.
Messa et al. 22 observed that the drug is also active NF-KB inhibitor in MDS, suggesting a possible role in reducing the transcription of antiapoptotic factor that may have an effect on erythroid inefficacy. Ghoti et al. 23 noted that deferasirox reduce iron overload directly and through elevation of hepcidin hormone. Interestingly, Ohyashiki et al. 24 demonstrated that deferasirox represses signaling through mTOR in myeloid leukemia cells.
There is evidence from retrospective studies that ICT may improve overall survival (OS) 9-17. At the present time, it is very difficult separate the prognostic impact of iron overload from the unfavorable impact of transfusion dependence as a biologic marker of bone marrow disease.
Regarding biochemistry parameters, transferrin saturation (TRS) and serum ferritin were studied during the 3-year follow-up. Indeed, they are simple tests, sensitive but not specific. Ferritin is also an acute-phase reactant and TRS has a day-to-day variability 18.
It is well recognized that red cell transfusion iron first accumulates within reticuloendothelial cell, thereafter macrophages release excess iron and organ damage occurred (liver, heart, endocrine glands). More accurate assessment of liver iron concentration (LIC) is possible via invasive methods (contraindicated in MDS due to thrombocytopenia) or via magnetic resonance imaging 18.
Several consensus groups have suggested guidelines on ICT 25-32 including parameters for initiation (20–25 RBC units and serum ferritin >1000 or >2000 ng/mL), patient features (life expectancy >6 months or >1 year and low/intermediate1 IPSS), target serum ferritin (<500 or <1000). Few retrospective studies assessed organ overload in MDS 9-17. In addition, cardiac iron disease is reported only in a small fraction of heavily transfused MDS 33-35.
Therefore, in the absence of prospective clinical trial iron chelation should be tailored to individuals. Life expectancy and comorbidities (especially renal impairment due to documented toxicity of the drug), accurate transfusion history and hepatitis (which may affect the pharmacokinetics of the drug), serum ferritin or laboratory evidence of iron excess (e.g., hereditary hemocromatosis) may represent key factors to make decisions about ICT.
Comparing different series of patients is difficult because of different duration of treatment or combination of iron chelation drugs, inclusion or exclusion of cases receiving other hematological modifying drugs, different decision making regarding red cell transfusion and response criteria. Principal clinical studies with deferasirox in MDS are summarized in Table 1 9-17.
| Study | Type | Efficacy | Results |
|---|---|---|---|
| Porter J. 9 | 1-year prospective phase II trial MDS (n = 47) and other rare anemias | Similar pattern of dose-dependent iron excretion and manageable safety profile | Changes in serum ferritin and LIC were correlated |
| Gatterman N. 10 | 1-year prospective phase IIIb trial MDS (n = 341) | Overall median serum ferritin decreased significantly during observation | Sustained reductions in LPI |
| Rose C. 11 | Retrospective and prospective analysis (2.5 years) MDS (n = 97) | Median OS was 53 and 124 months in nonchelated and in chelated ps | |
| Gatterman N. 12 | A post hoc analysis of EPIC trial MDS (n = 341) | Hematologic improvement was observed |
E/P/N responses were observed in 21% 13% and 22% of ps. Relapses rates for Hb/N/P responders were 40% 18.2% 7.7% |
| List A. 13 | 3-year prospective phase II trial MDS (n = 173) | For patients who completed 3 years on study, the median decrease in serum ferritin from baseline was 36% | E/P/N responses were observed in 15% 22% 15% of ps |
| Neukirchen J. 14 | A retrospective matched-pair analysis (n = 188) 94 ps on long-term ICT and 94 ps without ICT | Median OS was 49 and 74 months in nonchelated and in chelated ps | |
| Nolte F. 15 | 1-year prospective trial MDS (n = 50) | Hematologic improvement was observed | E/P/N responses were observed in 6% 30% 17% of ps |
| Angelucci E. 17 | A prospective trial MDS (n = 152) | Hematologic improvement was observed | E/N/P response was observed in 11% 3% 15% of ps |
| Lyons RM. 16 | A 24-month data from an ongoing, 5-year registry of lower risk MDS ps (n = 600) | Patients who received other treatment potentially active on MDS were reported | ICT was associated with longer median OS (52.2 months vs. 104.4 months) |
- LIC, liver iron concentration; LPI, labile plasma iron; OS, Overall Survival; E/P/N, erythroid/platelet/neutrophil; Hb, hemoglobin; ps, patients; ICT, iron chelation therapy.
Porter et al. compare the response of iron chelation in MDS and other anemias (Diamon-Blackfan, B-thalassemia). Similar manageable profile and efficacy were observed in different type of diseases. Furthermore, the changes in serum ferritin level correlated with change in LIC suggesting the benefit of regular serum ferritin monitoring during treatment 23. Gatterman et al. in the large 1-year EPIC study demonstrated that ICL670 was safe and effective in reducing iron burden and LPI. Serum ferritin level decreased following 12 months of ICT as confirmed more recently by Angelucci et al. 17.
Conclusions
Deferasirox, may represent an effective drug in lower risk MDS after other treatment options. In this experience, such as in literature, serum ferritin level decreased progressively over time and transfusion independence was obtained and maintained. Reduction in ferritin levels was anticipated by a decreased transfusion requirement suggesting a possible positive effect of the drug on hematopoiesis.
Reduction in oxidative stress may be responsible of the hematologic improvement but further possible explanation may impact on its benefit. The reasons of erythroid relapse remain unclear. Interestingly, major platelet response and ferritin decrease continue until the last follow-up.
Toxicity and increased morbidity due to iron overload are well-recognized complications associated with hemoglobin disorders, thalassemia (TDT), and sickle cell disease (SCD). Deferasirox is once-daily oral chelator approved by the Food and Drug Administration and the European Medicine Agency for use in transfusion-dependent anemias (TDT, SCD and MDS) and nontransfusion-dependent thalassemia. The experience with TDT is partially applicable to MDS due to different pattern of tissue iron distribution, age and comorbidities, genetic instability. Furthermore, some high-risk MDS patients do not survive long enough to accumulate iron organ damage 36, 37.
More biological and clinical studies are necessary to better define mechanisms of action of deferasirox and predicting factors of response 38, 39. Association of iron chelation therapy with other MDS modifying drugs or support strategies may increase rates and duration of response, but accurate assessment of toxicities and a longer follow-up are mandatory due to moderate or elevate discontinuation rate (40–80%), drug adverse events reported in these patients of over 60 years of age (gastrointestinal and renal episodes mainly): Table 2.
| Study | Characteristics | Extrahematological toxicities | Discontinuation | Hematological toxicities and death |
|---|---|---|---|---|
| Porter J. 9 |
MDS (n = 47). Mean age 65. Discontinuation rate 38% |
The most common AEs were gastrointestinal events and skin rash. No patient developed a serum creatinine level >2 times the ULN. Patient developed elevated ALT level (>5 times the ULN): 7.6% (n = 14) |
Discontinuation were due to increased serum creatinine 2.1% (n = 4). No patients discontinued drug because of increases in transaminase levels |
No hematological toxicities due to drug were reported. No death related to drug was reported |
| Gatterman N. 10 |
MDS (n = 341). Mean age 68. Discontinuation rate 49% |
The most common drug AEs were diarrhea, other gastrointestinal events and skin rash. One patient developed elevated ALT level (>10 times the ULN) |
Discontinuation as a result drug AEs 13% (n = 44), GI AEs 7% (n = 25). One patient discontinued drug because of transaminases. One acute renal failure was drug-related |
No hematological toxicities due to drug were reported. No death related to study drug was reported |
| List A. 13 |
MDS (n = 173). Mean age 71. Discontinuation rate 80% |
The most common moderate to severe drug AEs are GI events, blood creatinine increase |
Drug discontinuation as a result of AEs occurred in 25% (n = 43). Laboratory abnormalities led drug discontinuation 13.5% |
No death related to study drug were reported |
| Nolte F. 15 |
MDS (n = 50). Median age 69. Discontinuation rate 52% |
Most frequent drug EAs are GI. GI were mild and did not lead to drug discontinuation | Drug discontinuation as a result of increased creatinine 7% | No death related to study drug were reported |
| Angelucci E. 17 |
MDS (n = 152). Median age 72. Discontinuation rate 55% |
The most common moderate to severe drug AEs are GI, blood creatinine increase | Drug discontinuation as a result of drug AEs 33.3% (n = 28) | No death related to study drug was reported |
- AE, adverse events; ps, patients; MDS, myelodysplastic syndrome; GI, gastrointestinal; ULN, upper limit normal.
The severity of iron overload in MDS is a complex entity which includes RBC units, serum ferritin level, and organ dysfunction therefore there is a clinical need for a quantitative, noninvasive tool for iron measurement in this subset of elderly and cytopenic individuals.
A prospective randomized phase III trial, TELESTO, is enrolling patients to answer question of the role of deferasirox in lower risk myelodysplastic syndromes and its impact on survival.
In addition, there are interesting phase II trials ongoing: a phase II study of deferasirox and erythropoietin in low- and int-1-risk MDS patients and AZA plus ICL670 and/or Vitamin D in higher risk MDS.
Acknowledgments
The author thanks Dr Michele Centra the Director of Division of Immunohematology and Transfusion Medicine of “Riuniti” University Hospital Foggia (Italy) for his critical review of the manuscript, Peer Reviews and Editorial Board of the Journal for their valuable comments and assistance, all colleagues from Pesaro and Foggia Institutions for discussions that served to strengthen the paper.
Conflicts of Interest
The author declares no conflict of interest. No writing assistance was utilized in the production of this manuscript.




