MLL-ELL fusion gene in an acute myelomonocytic leukemia patient transformed from acute promyelocytic leukemia
Key Clinical Message
We report an extremely rare case of acute myelomonocytic leukemia (M4) with an MLL-ELL fusion gene lacking the PML-RARα rearrangement that transformed from hypergranular acute promyelocytic leukemia (APL) without showing any karyotypic evolution. The treatment was effective with chemotherapy for M4 and idarubicin plus a cytarabine-based chemotherapy protocol without ATRA.
Introduction
APL is a distinct disease characterized by the presence of the PML-RARα fusion gene. APL is prone to disseminated intravascular coagulation (DIC); however, it has a remarkable response to treatment with ATRA and arsenic trioxide (As2O3). APL may be recognized by different morphological presentations according to the type of granules present: (1) Hypergranular APL; (2) Microgranular APL; (3) Variant APL. The “variant” form of APL is characterized by cells with sparse or no granules and can easily be confused with acute monoblastic leukemia (M5) or M4. To distinguish variant APL cases from classical APL cases or other forms of AML, combining morphologic, cytochemical, immunophenotypic, cytogenetic, and molecular studies of leukemia-associated genes is necessary. Therapy-related APL (t-APL) has been increasingly reported recently in patients with malignancies 1. The most frequently reported primary neoplasms before t-APL are breast cancer, tumors of the autoimmune system and non-Hodgkin lymphoma 1. In most cases occurring after a primary tumor, the previous treatment consisted of topoisomerase II alpha (TOPOIIA) inhibitors 1. However, the transformation of APL into M4 after chemotherapy is extremely uncommon. Unlike the report by Todisco et al. 2, a different gene abnormality was found to develop in a patient treated for APL. Here, we report an extremely rare case of M4 with an MLL-ELL fusion gene lacking the PML-RARα rearrangement that transformed from hypergranular APL without showing any karyotypic evolution. Chemotherapy for M4 and idarubicin plus cytarabine-based chemotherapy protocol without ATRA was effective.
Case History/Examination
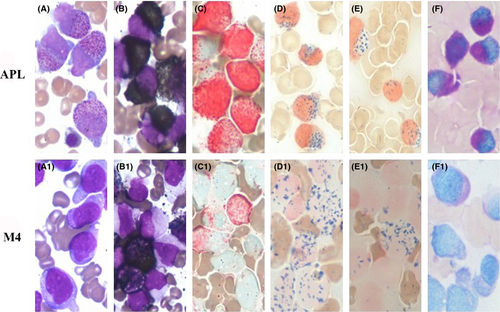
In April 2011, a 40-year-old man was admitted to our hospital because of gingival bleeding. Complete blood counts showed pancytopenia with white blood cells (WBC) 2.56 × 109/L (32% promyelocytes), 102 g/L hemoglobin (Hb), and a platelet count (Plt) of 13 × 109/L. Clinical and laboratory signs indicated coagulopathy. Bone marrow aspiration revealed that 83.2% of the nucleated cells were promyelocytes (Fig. 1). Immunophenotype analysis was compatible with a diagnosis of APL. The PML-RARα fusion gene (short-type) analysis was conclusive for a diagnosis of APL accompanied by DIC. There was no evidence of a family history of blood cancer. The patient was treated according to the Chinese guidelines for the diagnosis and treatment of acute promyelocytic leukemia (2011 Version). Briefly, the treatment was based on an all-trans retinoic acid (ATRA) plus anthracycline protocol (Table 1). The patient developed pancytopenia with hematological recovery after 32 days. Bone marrow aspiration showed a hematological complete remission (CR) was achieved. The patient then received consolidation courses every month. During the induction therapy, a repeat bone marrow aspiration showed regenerating marrow with no morphologic evidence of disease. After the last consolidation courses (approximately 2 months later), the patient presented with fever (39.5°C) and elevated WBC counts with juvenile cells (22.92 × 109/L; 15% juvenile cells). Morphology, cytochemical staining, molecular studies, cytogenetic and immunophenotype analyses were conclusive for a diagnosis of acute leukemia relapse (Fig. 1). The patient was diagnosed with M4 leukemia transformed from APL and treated with an idarubicin (8 mg/m2 per day for 2 days) and cytarabine (150 mg/m2 per day for 5 days) chemotherapy protocol (IA protocol). After the induction phase, the bone marrow aspirate revealed hematological CR. During the hematological CR, the patient attended Rui Jin Hospital Shanghai Jiao Tong University School of Medicine for further treatment. The present study adhered to the tenets of the Declaration of Helsinki.
| Course | Drug | Dosage | Days |
|---|---|---|---|
| 1a | ATRAc | 20 mg/m2 | 1–15 |
| IDAd | 8 mg/m2 | 1, 3, 5, 7 | |
| 2b | ATRAc | 20 mg/m2 | 1–15 |
| IDAd | 8 mg/m2 | 1–3 | |
| 3b | NVTd | 5 mg/m2 | 1–5 |
| ATRAc | 20 mg/m2 | 1–15 | |
| 4b | ATRAc | 20 mg/m2 | 1–15 |
| IDAd | 8 mg/m2 | 1–3 | |
| 5b | ATRAc | 20 mg/m2 | 1–15 |
| IDAd | 8 mg/m2 | 1–2 | |
| 6b | ATRAc | 20 mg/m2 | 1–15 |
| 7b | As2O3d | 0.16 mg/kg | 1–14 |
| 8b | As2O3d | 0.16 mg/kg | 1–14 |
- a Induction regimen.
- b Consolidation course.
- c Oral medicine.
- d Intravenous injection.
Flow Cytometry Analysis
Immunological studies were completed on admission using multiparameter flow cytometry and showed positivity with CD13, CD33, CD15, CD11b, CD117, and CD71 myeloid antigens; the patient was negative for HLA-DR and CD34. The leukemic cells expressed CD13, CD15, CD33, CD11b, and CD14 after relapse.
Cytogenetic Analysis and Molecular Studies
The fusion gene was detected by real-time quantitative polymerase chain reaction (RQ-PCR). Molecular studies showed the overexpression of the short form of the PML-RARα fusion gene and the EVI1 gene at onset (Table 2). As the disease becomes worse, the PML-RARα fusion gene and the retinoid acid receptor rearrangement were not detected by RQ-PCR. Abnormal expression and gene mutations in other leukemia-associated fusion genes were detected in the progression from APL to secondary M4. Characteristics of the genes are given in Table 2. In contrast to the APL gene mutation, a variant form of the MLL-ELL fusion transcript with t(11;19)(q23;p13) was observed in M4. Chromosome analysis with a G-banded karyotype of the unstimulated bone marrow cells showed 46, XY, in all 24 metaphase spreads, and no structural or numerical abnormalities were detected.
| Gene | Result | Gene | Result | ||
|---|---|---|---|---|---|
| APL | M4 | APL | M4 | ||
| MLL/AF6 | − | − | MLL/AF9 | − | − |
| MLL/AF10 | − | − | MLL/AF17 | − | − |
| MLL/ELL | − | + | DupMLL | − | − |
| AML1/ETO | − | − | PML/RARα | + | − |
| PLZF/RARα | − | − | NPM1/RARα | − | − |
| CBFB/MYH11 | − | − | NPM/MLF1 | − | − |
| TLS/ERG | − | − | DEK/CAN | − | − |
| EVI1 | + | + | HOX11 | − | − |
| FLT3/ITD | − | − | |||
- −: not detected; +: detected.
Discussion
APL is often curable and the prognosis is good for patients. However, our case experienced hematological relapse after being treated with anthracycline and an ATRA-based chemotherapy protocol.
To distinguish variant APL cases from classical APL cases or AML, combining morphologic and molecular studies of the PML-RARα or RARα-related fusion genes before chemotherapy is important. Although t(15;17) is almost always associated with APL, a small proportion of patients with APL have simple or complex variants of this translocation. These variant chromosomal translocations include t(11;17)(q23;q21) and t(5;17)(q35;q21), leading to PLZF-RARα and NPM1-RARα fusions 3, respectively. In the M4 case, early experiments did not detect any signs of PML-RARα (including bcr1, 2, 3) fusion or PLZF-RARα and NPM1-RARα fusions (Table 2). Chromosome analysis of the unstimulated bone marrow cells showed 46, XY, and no structural or numerical abnormalities were detected. Variant APL cases usually show different morphological pictures 4 and have also been reported to have leukemic cells with strong myeloperoxidase (MPO) activity 5. However, those results from laboratory experiments seem to conflict with the biologic and clinical characteristics of variant APL.
Therapy-related acute myeloid leukemia (t-AML) is a well-recognized complication induced by therapy given for the primary disease. The patient may receive several chemotherapy protocols during the consolidation phase, cumulative evidence indicates that among the different types of chemotherapy drugs, t-AML is always associated with TOPOIIA inhibitors (such as mitoxantrone and daunorubicin) 1, but there was no significant increase in the prevalence of t-AML after alternative chemotherapeutic approaches 6. In our case, the previous chemotherapy regimen included a TOPOIIA inhibitor. t-AML is thought to arise via the selection of a lymphoid/myeloid clone that has a significantly elevated risk for a mutational event. In abnormal cells, the DNA repair machinery is unable to rectify transient DNA breaks induced by TOPOIIA. These changes are related to impaired myeloid differentiation and the inhibition apoptosis, leading to leukemic transformation 1.
Sequence analysis of the RQ-PCR product revealed a novel form of MLL-ELL transcript in M4. The MLL-ELL fusion gene has been associated with many different types of hematological malignancies, especially AML 7. A total of 21.1% of all characterized MLL fusion genes were MLL-ELL 8. Unlike the primary hematopoietic tumor of our case, Kakihana et al. 9 reported a case of chronic myelomonocytic leukemia (CMML) with a 46, XY, t(11;19)(q23;p13.1) karyotype that transformed to AML. Lavau et al. 10 demonstrated the enhancing effect of MLL-ELL on the proliferative potential of hematopoietic progenitor cells as well as its causal role in the genesis of AML. In a mouse experiment, the expression of the MLL-ELL fusion protein seems to be sufficient as an initiating event to transform primary hematopoietic cells.
Moreover, blast cells express a variety of monocytic-associated antigens such as CD14 and CD11b, which are strongly associated with M5 and M4. In our case, the MLL-ELL fusion gene did not interfere with the clinical response to the therapy, and the patient had a good response to the conventional IA chemotherapy protocol without ATRA.
So, when combining morphological, cytochemical, immunophenotypic, cytogenetic, and molecular studies, we considered a variant form of the MLL-ELL fusion transcript with t(11;19)(q23; p13.1) in AML-M4 transformed from APL at diagnosis. Thus, a possibility is raised that the rare clinical presentation of the present case with the MLL-ELL fusion gene might represent a high risk for leukemic progression.
Acknowledgments
We gratefully acknowledge the patient for their participation in our study. We thank Dr. Xiao Bo Nie for the critical reading of this manuscript.
Conflict of Interest
The authors declare that the paper is the authors’ original work, has not been submitted to or published by another journal, and there are no competing financial interests or conflicts of interest in association with this research.




