Treatment of chronic lymphocytic leukemia with bendamustine in an HIV-infected patient on antiretroviral therapy: a case report and review of the literature
Key Clinical Message
Few reports have described the coincidence of chronic lymphocytic leukemia (CLL) and HIV. We administered bendamustine to an HIV-positive refractory CLL patient and obtained a significant objective response. Our results indicate that bendamustine can be used in HIV-infected CLL patients. We also reviewed 12 cases of CLL with HIV infection.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common form of adult leukemia in the Western world 1. Fludarabine has been reported to produce higher response rates in CLL, but not to affect overall survival 2. Furthermore, patients with CLL refractory to fludarabine have very poor outcomes 3. Therefore, salvage treatment against advanced refractory CLL is required. Bendamustine is an alternative agent with both alkylating and purine analog activities and is associated with a high rate of remission of CLL 4. The most commonly observed toxicities with bendamustine are myelosuppression and infections, including opportunistic infections such as cytomegalovirus 4. As patients with CLL tend to be highly immunosuppressed and prone to infection 5, the recommended dose of bendamustine for CLL is lower and the interval between sessions is longer than for non-Hodgkin's lymphoma 6.
Because of the advent of antiretroviral treatment (ART), the spectrum of morbidity and mortality of HIV-infected patients has improved dramatically. However, the risk of non-AIDS-defining malignancies in an HIV-infected population, including Hodgkin's lymphoma and some types of carcinoma 7, remains higher than that in the general population. Although CLL is a non-AIDS-defining malignancy and is common in Western countries, few reports have described cases of HIV concurrent with CLL in detail 8-18. Given that CLL patients are often immunocompromised and bendamustine has potent myelosuppressive activity, bendamustine treatment of CLL patients with concurrent HIV infection is associated with a higher risk of life-threatening opportunistic infections. We successfully administered bendamustine to a CLL patient with HIV infection and achieved significant clinical control of both diseases without occurrence of lethal infections.
Case Report
A 61-year-old heterosexual male developed anemia in December 2005. The patient's cervical, axillary, and inguinal lymph nodes were swollen in February 2006. The patient's white blood cell (WBC) count was 5.3 × 109/L (71.0% lymphocytes), the hemoglobin level was 52 g/L, and the platelet count was 123 × 109/L. A cervical lymph node biopsy revealed atypical lymphocyte infiltration, including a CD5+, CD11c+, CD19+, CD23+, and IgK-positive phenotype on immunological staining and flow cytometry. CD20 was slightly positive. G-banding analysis showed normal karyotypes (46, XY). Bone marrow (BM) infiltration was demonstrated by aspiration biopsy and the tumor cells were CD38+ phenotype. Fluorescent in situ hybridization using a chromosome 12 alpha-centromeric probe (D12Z3) did not detect trisomy 12. The patient was diagnosed with B-CLL (Rai III, Binet C), accompanied by Coombs-positive hemolytic anemia, lymphocytosis, and lymphadenopathy. Fludarabine therapy was started in March 2006. Rapidly, the anemia improved and cervical lymphadenopathy shrank. However, anti-HIV antibodies were detected in April 2006 after one course of fludarabine infusion (HIV-RNA level 4.6 × 105copies/mL, CD4+ cells 252/μL), and the patient was referred to our hospital in May 2006. In September, the progression of HIV infection (HIV-RNA level 6.1 × 105copies/mL, CD4+ cells 26/μL) was identified and ART (LPV/r + 3TC) was started. After four courses of fludarabine therapy, the anemia improved beyond 10 g/dL. However, he developed systemic lymphadenopathy, night sweats, and fatigue in August 2007. The soluble interleukin-2 receptor (sIL-2R) level was first estimated as 1640 U/mL in September. On the other hand, HIV was well controlled (HIV-RNA level<50 copies/mL, CD4+ cells 474/μL). In October, one course of fludarabine-therapy was added but anemia and lymphadenopathy was not improved. Therefore, two courses of rituximab plus fludarabine therapy were administered from November. We observed the decrease in peripheral lymphocytes and slight shrinkage of lymphadenopathy, however, anemia was not improved and sIL2-R level increased to 1980 U/mL in January 2008. We considered that CLL was refractory to fludarabine-based therapies. Therefore, eight courses of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) therapy were administered from January 2008, and subsequently six courses of rituximab plus cyclophosphamide, vincristine, and prednisone (R-COP) started from February 2010 using lymphademopathy and anemia symptoms as indexes. During the courses clinical symptoms were well controlled and no severe infection was observed. Although fatigue consisted, lymphadenopathy was improved and outpatient care became possible. The value of sIL-2R decreased to 1390 U/mL in February 2011.
In May 2011, the patient complained of constipation, a sense of abdominal distension, and rapid regrowth of the systemic lymph nodes. Eastern Cooperative Oncology Group (ECOG) performance status (PS) was estimated as 1. A physical examination revealed distinct systemic lymphadenopathy, hepatomegaly, and splenomegaly. He also felt short of breath and fatigue. The patient's WBC count was 12.2 × 109/L (66.0% lymphocytes; 3.4% atypical lymphocytes; and CD4+ lymphocytes, 742/μL), the hemoglobin level was 75 g/L, and the platelet count was 34 × 109/L. The sIL-2R level was 4370 U/mL. No HIV-RNA was detected with the real-time polymerase chain reaction. A BM aspiration revealed a dry tap, whereas a BM needle biopsy demonstrated hypercellular marrow with the increase in small lymphocytes (Fig. 1A). Immunohistochemical staining showed that the BM lymphocytes were CD5 + /CD20 + , compatible with CLL (Fig. 1B and C). A systemic computed tomography (CT) survey detected multiple lymphadenopathies as well as hepatosplenomegaly. The maximum diameter of the paraaortic lymph nodes was 8 cm (Fig. 2A).

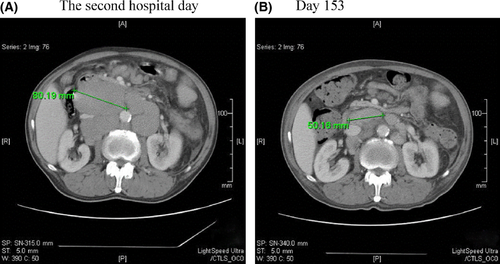
We diagnosed a relapse of CLL based on the increased lymphocyte count and sIL-2R level in peripheral blood. As the patient's disease was refractory to the previous chemotherapies, bendamustine was administered at a dose of 70 mg/m2/day for 2 days and this was repeated on days 42–43, 98–99, and 182–183 19 when he was in good physical condition after the delayed myeloid recovery plateaued (Fig. 3B), and considering his HIV infection. The patient's initial signs and symptoms such as constipation and abdominal distension improved remarkably by day 11. The superficial lymph nodes were well controlled during hospitalization. Because physical and hematological status was stable from day 78 to 97, he could intermittently stay at home in the period. The diameter of the paraaortic lymph nodes decreased to 5 cm on CT after three bendamustine sessions (Fig. 2B). While the hepatomegaly remained unchanged, the patient's spleen shrank slightly after each bendamustine infusion; however, it enlarged to 10 cm below the costal margin after the fourth course of bendamustine (Fig. 3A). The patient's peripheral lymphocyte count decreased rapidly after the first bendamustine infusion (Fig. 3A), although the tumor cells in his BM did not change significantly throughout the chemotherapy (Fig. 1A and D). After two courses of bendamustine treatment, the morphology of the tumor cells in the BM changed from a small phenotype before treatment to a medium-sized, morphologically atypical one. Immunological staining displayed CD5- and CD20+ (weak) phenotypes (Fig. 1D‒F). The sIL-2R level also decreased to 1770 U/mL (day 123) by the third bendamustine course, but ultimately increased to 6840 U/mL (Fig. 3A).
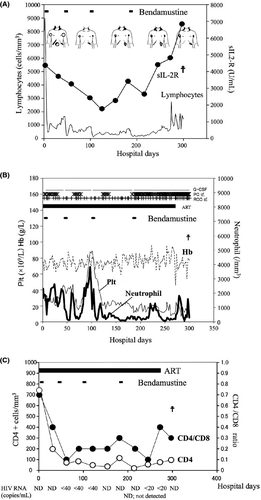
Anemia, thrombocytopenia, neutropenia, and febrile neutropenia were observed as CTC grade 4 adverse events. Thus, red blood cell and platelet transfusions were required during the patient's hospital stay. However, anemia symptoms such as fatigue and short of breath were not observed during bendamustine treatment. After the fourth course of bendamustine, pancytopenia progressed, and we stopped bendamustine treatment. During treatment, the patient developed hypercalcemia (11.7 mg/dL, day 41). Bisphosphonate and elcatonin were given, but they were terminated because the patient developed probable bisphosphonate-related osteonecrosis of the jaw (BRONJ). Performance status became worse (ECOG PS from 3 to 4) on day 268 and ingestion was impossible on day 270. He became somnolent on day 272. Subsequently, the patient's serum calcium concentration increased rapidly and reached 19.1 mg/dL. On day 299, the patient died of respiratory failure with multiorgan failure. Our patient survived for 78.5 months after starting fludarabine treatment and for 10.7 months after initiating bendamustine treatment. The treatment response was evaluated as stable disease (SD) based on the findings described above 20.
The HIV-RNA level was below the detection limit at hospitalization under ART consisting of abacavir (ABC)/lamivudine (3TC) plus lopinavir (LPV)/ritonavir(r). After the first bendamustine infusion, both the CD4+ cell number and CD4/CD8 ratio decreased rapidly. His CD4+ cell count had been below 100 cells/mm3 since day 60 (Fig. 3C). LPV/r was changed to raltegravir (RAL) on day 61 because of diarrhea. ART was completed on day 274 because oral administration was difficult due to the BRONJ-related pain of the jaw. Throughout the period of hospitalization, the HIV-RNA level was below the detection or quantitative limit (Fig. 3C). Trimethoprim-sulfamethoxazole (TMP-SMX) and azithromycin had been administered for the prevention of Pneumocystis jirovecii pneumonia and Mycobacterium avium complex infection, respectively, and no lethal opportunistic infection was observed. CMV pp65 antigenemia in the sera was also positive on days 46, 68, and 228, but became negative within 1 week with careful monitoring.
Discussion
The most important point in this report is the efficacy and safety of bendamustine treatment for CLL and a concurrent HIV infection treated with ART. Bendamustine demonstrated a significant antitumor effect using the recommended dose. In this case, bendamustine treatment carried a serious risk of infection because of its myelosuppressive properties and the patient's HIV status. However, potent myelosuppression did not lead to lethal opportunistic infections using prophylaxis for common infections. Furthermore, no HIV activation was observed using ART. To our knowledge, this is the first report describing the clinical course of refractory CLL with HIV infection treated with bendamustine alone. Our findings indicate that bendamustine might be a safe and effective treatment for severely immunocompromised patients.
Several articles have reported the efficacy of bendamustine for refractory CLL. Bergmann et al. 19 demonstrated that the response rate of bendamustine for relapsed or refractory CLL was 56% (complete remission, 2/16; partial remission, 5/16; and stable disease, 2/16), and the median overall survival time was 45.6 months. According to Kath et al. 21, the objective response rate against advanced, refractory, or relapsed CLL was 75% (complete remission, 6/20; partial remission, 9/20), and the median overall survival time was 13.6 months. A simple comparison of the present case with those studies was difficult because the protocols were not identical to that in our case. Considering that those studies were clinical trials performed under stringent patient eligibility criteria, the efficacy in the present case was notable. Bendamustine palliated physical sufferings such as constipation and abled the patient to stay at home, even if only short period. Lack of anemia symptoms during bendamustine treatment were probably due to appropriate transfusion and the rest state by hospitalization. Because the efficacy of bendamustine led to improvement of his quality of life as well as life prolongation, we consider that bendamustine treatment against refractory CLL was relevant.
A previous study demonstrated that bendamustine induced apoptosis on peripheral lymphocytes in B-CLL 22, suggesting that the reducing effect of bendamustine on the peripheral CLL cells, observed after initial infusion, was due to apoptosis. On the other hand, the lack of efficacy of bendamustine regarding BM CLL cells may be attributable to the survival benefit conferred by the microenvironment – the so called “niche” 23 – or to transformation of tumor cells, as in Richter's syndrome 24. Although confirmation of the former mechanism was difficult, pathological findings of BM tumor cells implied the latter mechanism. The observed morphological change (i.e., enlargement and heterogeneity of the tumor cells) suggests transformation to a diffuse large B-cell lymphoma (DLBCL)-like phenotype. Furthermore, the loss of CD5 was compatible with the immunological phenotype of Richter's syndrome. The CD38 + phenotype at diagnosis, a poor prognostic marker of CLL, might also have some effects on the disease progression.
Autoimmune hemolytic anemia (AIHA) with CLL 25 and thrombocytopenia due to HIV infection 26 might have contributed to the severe anemia and thrombocytopenia in this case, but the laboratory findings and the control of HIV infection do not support this possibility. Bendamustine is thought to be metabolized by CYP1A2 27, and its blood concentration is unlikely to be affected by the co-use of LPV/r, a potent CYP3A4 inhibitor 28; thus, we can exclude the possibility of bendamustine overdosage. Bendamustine also caused the decrease in the CD4+ cell counts and CD4/8 ratio, as has been reported elsewhere 21. CLL and HIV likely contributed to the abnormalities in the T-cell subset 5, 29; however, the patient's immunosuppression was managed using prophylaxis for P. jirovecii pneumonia, M. avium complex infection, and viral and fungal infections.
The co-occurrence of CLL/small lymphocytic lymphoma (SLL) and HIV is rare. Although several studies have described CLL/SLL with HIV infection (Table 1), most are exploratory and/or epidemiological surveys of AIDS-related lymphoma 8, 10, 11, 14, 16. Case number 11 in Table 1 is most likely adult T-cell leukemia because of the monoclonal integration of human T-lymphotropic virus type 1 DNA in peripheral white blood cells. One reason for the rarity of CLL and HIV co-occurrence is the difference in the typical affected age. CLL is frequently observed in elderly populations, but most HIV carriers and AIDS patients are of reproductive age 13. In addition, few reports describe the treatments used (Table 1). Only two reports have described treatment of B-CLL with HIV infection 13, 15. Cole et al. 15 reported four cases of CLL with HIV infection. Of those, case number 2 in Table 1 received treatment for untreated CLL using bendamustine with rituximab plus ART. The authors reported an objective response in the swelling lymph nodes and lymphocytosis without severe adverse effects; however, the dose and schedule of the bendamustine infusion was not described. Ravandi et al. 13 reported the treatment of a 65-year-old male with B-CLL occurring during the follow-up period of HIV infection (case number 5 in Table 1). The authors reported persistent BM infiltration similar to that observed in our case. They also observed a correlation between the CD4 + cell number and HIV viral load during chemotherapy. They hypothesized that the rapid and profound reduction in CD4 + T cells by chemotherapies depletes the available host cells for HIV viral replication, hindering viral propagation. The control of the HIV infection in our patient might be attributed to the same mechanism.
| Case number | Age | Sex | CD4/HIV-VL | HIV duration | Tx | Case reference |
|---|---|---|---|---|---|---|
| Present case | 61 | M | 252/4.6 × 105 | Simultaneous | Flu/R-Flu/R-C(H)OP/BEN | |
| 1 | 76 | M | 396/<75 | <1 year | no Tx | 15 |
| 2 | 68 | M | 1374/<75 | 9 years | BEN+R | 15 |
| 3 | 55 | M | 487/5297 | 10 years | Chlorambucil | 15 |
| 4 | 55 | F | 497/2965 | 15 years | Flu, CY, R | 15 |
| 5 | 65 | M | 4000/NA(>0) | 8 years | Chlorambucil, Flu, Campath1H | 13 |
| 6 | NA | NA (Two cases of 10 indolent non-Hodgikin lymphoma were described as SLL.) | 12 | |||
| 7 | NA | 12 | ||||
| 8 | 31 | 2M/1F NA (T-CLL: One was clinically AIDS and the other two were HIV seropositive.) | no Tx | 9 | ||
| 9 | 34 | no Tx | 9 | |||
| 10 | 35 | no Tx | 9 | |||
| 11 | 64 | M | 734/NA | Uncertain | no Tx | 8, 17 |
| 12 | 50 | M | 1100/NA | Simultaneous | no Tx | 18 |
- HIV-VL, HIV viral load; M, male; F, female; ND, not detected; NA, not acquired; Tx, treatment; Flu, Fludarabine; R, rituximab; C(H)OP, cyclophosphamide, (doxorubicin), vincristine, and prednisolone, BEN, bendamustine; CY, cyclophosphamide.
In conclusion, we introduced bendamustine in refractory B-CLL accompanied by HIV infection and observed a significant objective response with concurrent ART. Although myelosuppression was a major problem, no lethal opportunistic infection occurred with standard prophylaxis. In the future, the number of CLL/SLL patients with HIV infection will increase because of the widespread use of ART. Thus, further evaluation of the safety and efficacy of bendamustine against CLL/SLL with HIV infection is necessary.
Acknowledgments
We are grateful to Dr. Mieko Chinzei, Noriko Fujiwara, Reiko Yamahana, and Aya Watanabe (Palliative Care Team, Research Hospital, The Institute of Medical Science, the University of Tokyo, Tokyo, Japan) for providing excellent pain control to our patient.
Conflict of Interest
The authors declare no conflicts of interest. No assistance with the preparation of this manuscript was received.




