Advances and Strategies towards Synthesis of Aspidosperma Indole Alkaloids Goniomitine
Corresponding Author
Cong-Bin Ji
School of Chemistry and Environmental Sciences, Shangrao Normal University, 334001 Shangrao, P. R. China
Search for more papers by this authorCorresponding Author
Cong-Bin Ji
School of Chemistry and Environmental Sciences, Shangrao Normal University, 334001 Shangrao, P. R. China
Search for more papers by this authorAbstract
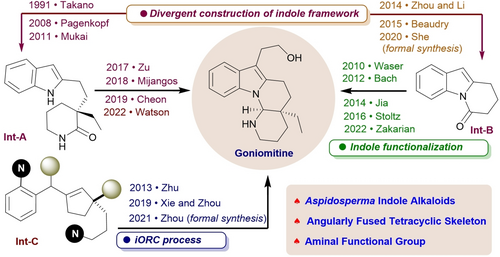
Goniomitine is of the aspidosperma alkaloid family, with an angularly fused tetracyclic skeleton housing an all-carbon quaternary carbon chiral center alongside an aminal functional group. This natural product has garnered attention as a synthetic target due to its intriguing molecular architecture and anti-proliferative activity in recent years. Following the first synthesis of (−)-goniomitine by Takano in 1991, synthetic chemists have developed various methods. This review provides an overview of the methodologies used in the synthesis of goniomitine in racemic and enantiopure forms via divergent construction indole framework, indole functionalization, and the integrated oxidation/reduction/cyclization (iORC) sequence from 1991 to 2023.
Graphical Abstract
Conflict of interests
The authors declare no conflict of interest.
References
- 1J. E. Saxton in The Alkaloids: Chemistry and Biology, Vol. 51 (Eds.: G. A. Cordell), Academic Press: San Diego, 1998, p. 1–197.
- 2A. Palmieri, M. Petrini, Nat. Prod. Rep. 2019, 36, 490–530.
- 3M. Ishikura, K. Yamada, T. Abe, Nat. Prod. Rep. 2010, 27, 1630–1680.
- 4J. Hájíček, Collect. Czech. Chem. Commun. 2011, 76, 2023–2083.
- 5J. C. Vantourout, A. Isidro-Llobet, A. J. B. Watson, in Studies in Natural Products Chemistry, Vol. 55 (Ed.: Atta-ur-Rahman FRS), Elsevier B. V., Amsterdam, 2018, p. 1–29.
- 6L. Randriambola, J.-C. Quirion, C. Kan-Fan, H.-P. Husson, Tetrahedron Lett. 1987, 28, 2123–2126.
- 7C. Hashimoto, H.-P. Husson, Tetrahedron Lett. 1988, 29, 4563–4566.
- 8J. Le Men, W. I. Taylor, Experientia 1965, 21, 508–510.
- 9S. Takano, T. Sato, K. Inomata, K. Ogasawara, J. Chem. Soc. Chem. Commun. 1991, 462–464.
- 10M. Mizutani, F. Inagaki, T. Nakanishi, C. Yanagihara, I. Tamai, C. Mukai, Org. Lett. 2011, 13, 1796–1799.
- 11F. De Simone, J. Gertsch, J. Waser, Angew. Chem. Int. Ed. 2010, 49, 5767–5770; Angew. Chem. 2010, 122, 5903–5906.
- 12Z. Xu, Q. Wang, J. Zhu, Chem. Soc. Rev. 2018, 47, 7882–7898.
- 13O. Mitsunobu, Synthesis 1981, 1981, 1–28.
- 14H. Li, P. Cheng, L. Jiang, J.-L. Yang, L. Zu, Angew. Chem. Int. Ed. 2017, 56, 2754–2757; Angew. Chem. 2017, 129, 2798–2801.
- 15T. Uyehara, N. Takehara, M. Ueno, T. Sato, Bull. Chem. Soc. Jpn. 1995, 68, 2687–2694.
- 16M. V. Mijangos, L. D. Miranda, Org. Biomol. Chem. 2018, 16, 9409–9419.
- 17H. K. Grover, M. A. Kerr, Synlett 2015, 26, 815–819.
- 18M. Yu, B. L. Pagenkopf, Tetrahedron 2005, 61, 321–347.
- 19F. Gnad, O. Reiser, Chem. Rev. 2003, 103, 1603–1624.
- 20H. Y. Reissig, R. Zimmer, Chem. Rev. 2003, 103, 1151–1196.
- 21M. Yu, B. L. Pagenkopf, J. Am. Chem. Soc. 2003, 125, 8122–8123.
- 22M. Yu, B. L. Pagenkopf, Org. Lett. 2003, 5, 5099–5101.
- 23M. Yu, G. D. Pantos, J. L. Sessler, B. L. Pagenkopf, Org. Lett. 2004, 6, 1057–1059.
- 24C. L. Morales, B. L. Pagenkopf, Org. Lett. 2008, 10, 157–159.
- 25C. Mukai, Y. Takahashi, Org. Lett. 2005, 7, 5793–5796.
- 26J. A. Marshall, X.-j. Wang, J. Org. Chem. 1990, 55, 6246–6248.
- 27J. A. Marshall, X.-j. Wang, J. Org. Chem. 1992, 57, 1242–1252.
- 28J. A. Marshall, C. M. Grant, J. Org. Chem. 1999, 64, 8214–8219.
- 29S. B. Garber, J. S. Kingsbury, B. L. Gray, A. H. Hoveyda, J. Am. Chem. Soc. 2000, 122, 8168–8179.
- 30B. Zhou, J. Du, Y. Yang, Y. Li, Chem. Eur. J. 2014, 20, 12768–12772.
- 31H. Yin, L. Xu, N. A. Porter, Chem. Rev. 2011, 111, 5944–5972.
- 32J. K. Vellucci, C. M. Beaudry, Org. Lett. 2015, 17, 4558–4560.
- 33R. Kim, A. J. Ferreira, C. M. Beaudry, Angew. Chem. Int. Ed. 2019, 58, 12595–12598;
Angew. Chem. 2019, 131, 12725–12728.
10.1002/ange.201907455 Google Scholar
- 34E. Parka, C.-H. Cheon, Adv. Synth. Catal. 2019, 361, 4888–4892.
- 35S.-J. Lee, H.-A. Seo, C.-H. Cheon, Adv. Synth. Catal. 2016, 358, 1566–1570.
- 36H.-A. Seo, C.-H. Cheon, J. Org. Chem. 2016, 81, 7917–7923.
- 37D. C. Behenna, Y. Liu, T. Yurino, J. Kim, D. E. White, S. C. Virgil, B. M. Stoltz, Nat. Chem. 2012, 4, 130–133.
- 38G. E. Bell, J. W. B. Fyfe, E. M. Israel, A. M. Z. Slawin, M. Campbell, A. J. B. Watson, Org. Lett. 2022, 24, 3024–3027.
- 39R. C. Larock, E. K. Yum, J. Am. Chem. Soc. 1991, 113, 6689–6690.
- 40L. Jiao, E. Herdtweck, T. Bach, J. Am. Chem. Soc. 2012, 134, 14563–14572.
- 41S. Zhou, Y. Jia, Org. Lett. 2014, 16, 3416–3418.
- 42G. Stork, K. Zhao, Tetrahedron Lett. 1989, 30, 2173–2174.
- 43B. P. Pritchett, J. Kikuchi, Y. Numajiri, B. M. Stoltz, Angew. Chem. Int. Ed. 2016, 55, 13529–13532; Angew. Chem. 2016, 128, 13727–13730.
- 44A. E. Strom, J. F. Hartwig, J. Org. Chem. 2013, 78, 8909–8914.
- 45Y. Li, E. Paola, Z. Wang, G. Menard, A. Zakarian, Angew. Chem. Int. Ed. 2022, 61, e202209987;
Angew. Chem. 2022, 134, e202209987.
10.1002/ange.202209987 Google Scholar
- 46S. Zhu, N. Niljianskul, S. L. Buchwald, J. Am. Chem. Soc. 2013, 135, 15746–15749.
- 47S. Zhu, S. L. Buchwald, J. Am. Chem. Soc. 2014, 136, 15913–15916.
- 48Y. Yang, S.-L. Shi, D. Niu, P. Liu, S. L. Buchwald, Science 2015, 349, 62–66.
- 49For a review: M. T. Pirnot, Y. M. Wang, S. L. Buchwald, Angew. Chem. Int. Ed. 2016, 55, 48–57.
- 50Z. Xu, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2013, 52, 3272–3276; Angew. Chem. 2013, 125, 3354–3358.
- 51O. Wagnières, Z. Xu, Q. Wang, J. Zhu, J. Am. Chem. Soc. 2014, 136, 15102–15108.
- 52H.-Y. Bin, K. Wang, D. Yang, X.-H. Yang, J.-H. Xie, Q.-L. Zhou, Angew. Chem. Int. Ed. 2019, 58, 1174–1177; Angew. Chem. 2019, 131, 1186–1189.
- 53F. Antonietti, E. Brenna, C. Fuganti, F. G. Gatti, T. Giovenzana, V. Grande, L. Malpezzi, Synthesis 2005, 2005, 1148–1156.
- 54F. Lebreux, F. Buzzo, I. Markó, Synlett 2008, 2008, 2815–2820.
- 55S. Ma, D. Long, P. Chen, H. Shi, H. Li, R. Fang, X. Wang, X. Xie, X. She, Org. Chem. Front. 2020, 7, 2689–2695.
- 56Z. Li, S. Ma, F. Liu, R. Ma, J. Zhao, X. Xie, X, She, Org. Biomol. Chem. 2022, 20, 6314–6318.
- 57K. Zhang, Q. Liu, R. He, D. Chen, Z. Deng, N. Huang, H. Zhou, Green Chem. 2021, 23, 1628–1632.
- 58W. Jiang, J. Bai, J. Lv, Y. Zhao, C. Yan, Z. Shi, Synlett 2023, 34, 2220–2226.