Identifying Anti-NSCLC Bioactive Compounds in Scutellaria via 2D NMR-Based Metabolomic Analysis of Pharmacologically Classified Crude Extracts
Jialuo Chen
State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137 China
School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, 611137 China
These authors contributed equally to this work.
Contribution: Data curation (lead), Formal analysis (lead), Methodology (lead), Software (lead), Writing - original draft (lead)
Search for more papers by this authorYanping Li
School of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
These authors contributed equally to this work.
Contribution: Methodology (equal), Software (equal)
Search for more papers by this authorXiu Gu
Innovative Institute of Chinese Medicine and Pharmacy, Chengdu University of Traditional Chinese Medicine, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
Contribution: Methodology (equal)
Search for more papers by this authorTianren Wu
State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137 China
School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, 611137 China
Search for more papers by this authorHuan Du
State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137 China
School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, 611137 China
Contribution: Software (equal)
Search for more papers by this authorCaihong Bai
School of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
Search for more papers by this authorCorresponding Author
Jiahui Yang
- [email protected]
- +86-28-61800219
School of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
Contribution: Funding acquisition (equal), Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
Kaifeng Hu
- [email protected]
- +86-28-61800087
State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137 China
Innovative Institute of Chinese Medicine and Pharmacy, Chengdu University of Traditional Chinese Medicine, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
Contribution: Funding acquisition (lead), Writing - review & editing (lead)
Search for more papers by this authorJialuo Chen
State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137 China
School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, 611137 China
These authors contributed equally to this work.
Contribution: Data curation (lead), Formal analysis (lead), Methodology (lead), Software (lead), Writing - original draft (lead)
Search for more papers by this authorYanping Li
School of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
These authors contributed equally to this work.
Contribution: Methodology (equal), Software (equal)
Search for more papers by this authorXiu Gu
Innovative Institute of Chinese Medicine and Pharmacy, Chengdu University of Traditional Chinese Medicine, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
Contribution: Methodology (equal)
Search for more papers by this authorTianren Wu
State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137 China
School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, 611137 China
Search for more papers by this authorHuan Du
State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137 China
School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, 611137 China
Contribution: Software (equal)
Search for more papers by this authorCaihong Bai
School of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
Search for more papers by this authorCorresponding Author
Jiahui Yang
- [email protected]
- +86-28-61800219
School of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
Contribution: Funding acquisition (equal), Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
Kaifeng Hu
- [email protected]
- +86-28-61800087
State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137 China
Innovative Institute of Chinese Medicine and Pharmacy, Chengdu University of Traditional Chinese Medicine, No.1166 Liutai Avenue, Chengdu, Sichuan, 611137 China Tel.
Contribution: Funding acquisition (lead), Writing - review & editing (lead)
Search for more papers by this authorAbstract
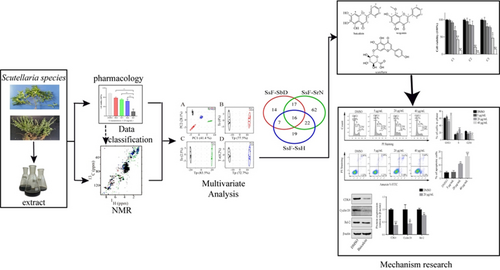
We presented a strategy utilizing 2D NMR-based metabolomic analysis of crude extracts, categorized by different pharmacological activities, to rapidly identify the primary bioactive components of TCM. It was applied to identify the potential bioactive components from Scutellaria crude extracts that exhibit anti-non-small cell lung cancer (anti-NSCLC) activity. Four Scutellaria species were chosen as the study subjects because of their close phylogenetic relationship, but their crude extracts exhibit significantly different anti-NSCLC activity. Cell proliferation assay was used to assess the anti-NSCLC activity of four species of Scutellaria. 1H−13C HSQC spectra were acquired for the chemical profiling of these crude extracts. Based on the pharmacological classification (PCA, OPLS-DA and univariate hypothesis test) were performed to identify the bioactive constituents in Scutellaria associated with the anti-NSCLC activity. As a result, three compounds, baicalein, wogonin and scutellarin were identified as bioactive compounds. The anti-NSCLC activity of the three potential active compounds were further confirmed via cell proliferation assay. The mechanism of the anti-NSCLC activity by these active constituents was further explored via flow cytometry and western blot analyses. This study demonstrated 2D NMR-based metabolomic analysis of pharmacologically classified crude extracts to be an efficient approach to the identification of active components of herbal medicine.
Graphical Abstract
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202400258-sup-0001-misc_information.pdf385.3 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Huang, R. Li, M. Yang, A. Zhou, H. Wu, Z. Li, H. Wu, Front. Pharmacol. 2022, 13, 989139.
- 2J. H. Wu, Y. T. Cao, H. Y. Pan, L. H. Wang, Molecules 2020, 25, 4269.
- 3W. W. Chen, K. K. Gong, L. J. Yang, J. J. Dai, Q. Zhang, F. Wang, X. L. Li, S. C. Xi, J. Du, J. Ethnopharmacol. 2021, 265, 113295.
- 4N. Bohni, M. L. Cordero-Maldonado, J. Maes, D. Siverio-Mota, L. Marcourt, S. Munck, A. R. Kamuhabwa, M. J. Moshi, C. V. Esguerra, P. A. de Witte, A. D. Crawford, J. L. Wolfender, PLoS One 2013, 8, e64006.
- 5S. F. Wang, H. Q. Wang, Y. N. Liu, Y. Wang, X. H. Fan, Y. Y. Cheng, Sci. Rep. 2016, 6, 3100.
- 6D. Lv, J. Xu, M. Qi, D. Wang, W. Xu, L. Qiu, Y. Li, Y. Cao, J. Pharm. Anal. 2022, 12, 500–508.
- 7S. H. Shi, Y. P. Cai, X. J. Cai, X. Y. Zheng, D. S. Cao, F. Q. Ye, Z. Xiang, PLoS One 2014, 9, e89123.
- 8A. H. Emwas, R. Roy, R. T. McKay, L. Tenori, E. Saccenti, G. A. N. Gowda, D. Raftery, F. Alahmari, L. Jaremko, M. Jaremko, D. S. Wishart, Metabolites 2019, 9, 123.
- 9Y. Pacheco-Hernández, D. Hidalgo-Martínez, G. Zepeda-Vallejo, Y. Cruz-Narváez, R. L. Escobar-García, E. Becerra-Martínez, N. Villa-Ruano, Chem. Biodiversity 2022, 19, e202200745.
- 10F. Tang, E. Hatzakis, Anal. Chem. 2020, 92, 11177–11185.
- 11M. A. Salem, L. Perez de Souza, A. Serag, A. R. Fernie, M. A. Farag, S. M. Ezzat, S. Alseekh, Metabolites 2020, 10, 37.
- 12I. Timari, C. Wang, A. L. Hansen, G. C. dos Santos, S. O. Yoon, L. Bruschweiler-Li, R. Bruschweiler, Anal. Chem. 2019, 91, 2304–2311.
- 13F. Puig-Castellvi, Y. Perez, B. Pina, R. Tauler, I. Alfonso, Anal. Chem. 2018, 90, 12422–12430.
- 14A.-H. Emwas, K. Szczepski, B. G. Poulson, K. Chandra, R. T. McKay, M. Dhahri, F. Alahmari, L. Jaremko, J. I. Lachowicz, M. Jaremko, Molecules 2020, 25, 4597.
- 15K. Chandra, S. Al-Harthi, F. Almulhim, A.-H. Emwas, Ł. Jaremko, M. Jaremko, Mol. Omics 2021, 17, 719–724.
- 16L. Zhu, S. J. Ma, M. J. Liu, K. L. Li, E. Shuai, Z. M. Wang, S. N. Li, S. L. Zhang, W. Cai, Front. Plant Sci. 2022, 13, 1012553.
- 17B. Li, H. Shao, L. Gao, H. Li, H. Sheng, L. Zhu, Drug Delivery 2022, 29, 2130–2161.
- 18M. Shah, S. Mubin, S. S. U. Hassan, P. Tagde, O. Ullah, M. H. Rahman, A. Al-Harrasi, N. U. Rehman, W. Murad, Biomol. Eng. 2022, 12, 936.
- 19J. Li, H. Wang, X. Shi, L. Zhao, T. Lv, Q. Yuan, W. Hao, J. Zhu, J. Ethnopharmacol. 2019, 235, 155–163.
- 20J. Shen, P. Li, S. Liu, Q. Liu, Y. Li, Y. Sun, C. He, P. Xiao, J. Ethnopharmacol. 2021, 265, 113198.
- 21D. Sheng, B. Zhao, W. Zhu, T. Wang, Y. Peng, BMC Complement. Med. Ther. 2022, 22, 120.
- 22J. Y. Li, D. Wang, P. P. Xue, H. R. Sun, Q. H. Feng, N. Miao, Mitochondr. DNA B 2021, 6, 84–85.
10.1080/23802359.2020.1847621 Google Scholar
- 23Q. T. Han, K. Xiao, K. L. Xiang, G. S. Li, S. J. Dai, Chem. Biodiversity 2018, 15, e1800038.
- 24X. Z. Zhu, C. X. Han, T. Gao, H. Shao, J. Essent. Oil-Bear. Plants 2016, 19, 664–670.
- 25X. Shang, X. He, X. He, M. Li, R. Zhang, P. Fan, Q. Zhang, Z. Jia, J. Ethnopharmacol. 2010, 128, 279–313.
- 26Q. Wang, N. Acharya, Z. Liu, X. Zhou, M. Cromie, J. Zhu, W. Gao, J. Ethnopharmacol. 2018, 217, 140–151.
- 27G. Su, H. Chen, X. Sun, Cancer Biomarkers 2018, 22, 13–18.
- 28C. Lu, H. Wang, S. Chen, R. Yang, H. Li, G. Zhang, J. Cell. Mol. Med. 2018, 22, 2478–2487.
- 29H. J. Kim, C. Park, M. H. Han, S. H. Hong, G. Y. Kim, S. Hoon Hong, N. Deuk Kim, Y. H. Choi, Drug Dev. Res. 2016, 77, 73–86.
- 30L. Chen, Y. Q. Luo, T. Liu, Y. H. Yuan, H. L. Gu, Y. Yang, L. Li, L. Tan, Talanta 2015, 132, 479–485.
- 31F. Delaglio, S. Grzesiek, G. W. Vuister, G. Zhu, J. Pfeifer, A. Bax, J. Biomol. NMR 1995, 6, 277–293.
- 32X. Gu, S. Zhu, H. Du, C. Bai, X. Duan, Y. Li, K. Hu, Microchem. J. 2022, 176, 107225.
- 33D.-W. Li, A. Leggett, L. Bruschweiler-Li, R. Brüschweiler, Anal. Chem. 2022, 94, 8674–8682.
- 34H. Du, X. Gu, J. Chen, C. Bai, X. Duan, K. Hu, Anal. Chem. 2023, 95, 3195–3203.
- 35J. Chong, O. Soufan, C. Li, I. Caraus, S. Li, G. Bourque, D. S. Wishart, J. Xia, Nucleic Acids Res. 2018, 46, W486–W494.
- 36J. Boccard, D. N. Rutledge, Anal. Chim. Acta 2013, 769, 30–39.
- 37K. Cho, Y. Kim, S. J. Wi, J. B. Seo, J. Kwon, J. H. Chung, K. Y. Park, M. H. Nam, J. Agric. Food Chem. 2012, 60, 11015–11028.
- 38T. Huang, P. Y. Chen, B. Liu, X. Li, X. Q. Lv, K. F. Hu, Anal. Chem. 2020, 92, 10996–11006.