Trichome Density in Relation to Volatiles Emission and 1,8-Cineole Synthase Gene Expression in Thymus albicans Vegetative and Reproductive Organs
Corresponding Author
Natália T. Marques
Centro de Eletrónica, Optoeletrónica e Telecomunicações, Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal
Search for more papers by this authorAlexandra Filipe
Núcleo de Biologia Comparativa e Integrativa, Centro de Ciências do Mar, Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal
Search for more papers by this authorPatrícia Pinto
Núcleo de Biologia Comparativa e Integrativa, Centro de Ciências do Mar, Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal
Search for more papers by this authorJosé Barroso
Centro de Estudos do Ambiente e do Mar (CESAM Lisboa), Faculdade de Ciências da Universidade de Lisboa, Centro de Biotecnologia Vegetal (CBV), DBV, C2, Piso 1, Campo Grande, 1749-016 Lisboa, Portugal
Search for more papers by this authorHelena Trindade
Centro de Estudos do Ambiente e do Mar (CESAM Lisboa), Faculdade de Ciências da Universidade de Lisboa, Centro de Biotecnologia Vegetal (CBV), DBV, C2, Piso 1, Campo Grande, 1749-016 Lisboa, Portugal
Search for more papers by this authorDeborah M. Power
Núcleo de Biologia Comparativa e Integrativa, Centro de Ciências do Mar, Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal
Search for more papers by this authorAna Cristina Figueiredo
Centro de Estudos do Ambiente e do Mar (CESAM Lisboa), Faculdade de Ciências da Universidade de Lisboa, Centro de Biotecnologia Vegetal (CBV), DBV, C2, Piso 1, Campo Grande, 1749-016 Lisboa, Portugal
Search for more papers by this authorCorresponding Author
Natália T. Marques
Centro de Eletrónica, Optoeletrónica e Telecomunicações, Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal
Search for more papers by this authorAlexandra Filipe
Núcleo de Biologia Comparativa e Integrativa, Centro de Ciências do Mar, Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal
Search for more papers by this authorPatrícia Pinto
Núcleo de Biologia Comparativa e Integrativa, Centro de Ciências do Mar, Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal
Search for more papers by this authorJosé Barroso
Centro de Estudos do Ambiente e do Mar (CESAM Lisboa), Faculdade de Ciências da Universidade de Lisboa, Centro de Biotecnologia Vegetal (CBV), DBV, C2, Piso 1, Campo Grande, 1749-016 Lisboa, Portugal
Search for more papers by this authorHelena Trindade
Centro de Estudos do Ambiente e do Mar (CESAM Lisboa), Faculdade de Ciências da Universidade de Lisboa, Centro de Biotecnologia Vegetal (CBV), DBV, C2, Piso 1, Campo Grande, 1749-016 Lisboa, Portugal
Search for more papers by this authorDeborah M. Power
Núcleo de Biologia Comparativa e Integrativa, Centro de Ciências do Mar, Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal
Search for more papers by this authorAna Cristina Figueiredo
Centro de Estudos do Ambiente e do Mar (CESAM Lisboa), Faculdade de Ciências da Universidade de Lisboa, Centro de Biotecnologia Vegetal (CBV), DBV, C2, Piso 1, Campo Grande, 1749-016 Lisboa, Portugal
Search for more papers by this authorAbstract
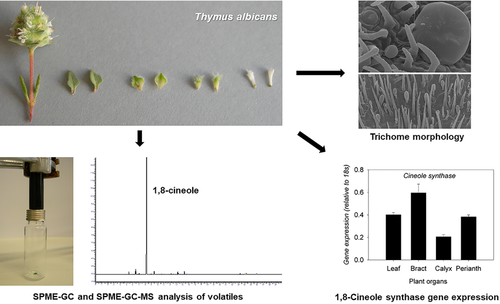
1,8-Cineole is the main volatile produced by Thymus albicans Hoffmanns. & Link 1,8-cineole chemotype. To understand the contribution of distinct plant organs to the high 1,8-cineole production, trichome morphology and density, as well as emitted volatiles and transcriptional expression of the 1,8-cineole synthase (CIN) gene were determined separately for T. albicans leaves, bracts, calyx, corolla and inflorescences. Scanning electron microscopy (SEM) and stereoscope microscopy observations showed the highest peltate trichome density in leaves and bracts, significantly distinct from calyx and corolla. T. albicans volatiles were collected by solid phase micro extraction (SPME) and analyzed by gas chromatography-mass spectrometry (GC/MS) and by GC for component identification and quantification, respectively. Of the 23 components identified, 1,8-cineole was the dominant volatile (57–93 %) in all T. albicans plant organs. The relative amounts of emitted volatiles clearly separated vegetative from reproductive organs. Gene expression of CIN was assigned to all organs analyzed and was consistent with the relatively high emission of 1,8-cineole in leaves and bracts. Further studies will be required to analyze monoterpenoid biosynthesis by each type of glandular trichome.
Graphical Abstract
References
- 1R. Morales, ‘The history, botany and taxonomy of the genus Thymus’, Taylor & Francis, London, 2002.
- 2J. Gershenzon, M. Maffei, R. Croteau, ‘Biochemical and histochemical localization of monoterpene biosynthesis in the glandular trichomes of spearmint (Mentha spicata)’, Plant Physiol. 1989, 89, 1351–1357.
- 3E. Werker, ‘Trichome diversity and development’, Adv. Bot. Res. 2000, 31, 1–35.
- 4M. D. Mendes, A. C. Figueiredo, M. M. Oliveira, H. Trindade, ‘Essential oil production in shoot cultures versus field-grown plants of Thymus caespititius’, Plant Cell Tissue Organ Cult. 2013, 113, 341–351.
- 5M. Majdi, A. Malekzadeh-Mashhady, A. Maroufi, C. Crocoll, ‘Tissue-specific gene-expression patterns of genes associated with thymol/carvacrol biosynthesis in thyme (Thymus vulgaris L.) and their differential changes upon treatment with abiotic elicitors’, Plant Physiol. Biochem. 2017, 115, 152–162.
- 6L. Ascensão, N. Marques, M. S. Pais, ‘Glandular trichomes on vegetative and reproductive organs of Leonotis leonurus (Lamiaceae)’, Ann. Bot. 1995, 75, 619–626.
- 7K. Xiao, X. Mao, Y. Lin, H. Xu, Y. Zhu, Q. Cai, H. Xie, J. Zhang, ‘Trichome, a functional diversity phenotype in plant’, Mol. Biol. 2017, 6, 183.
- 8A. Tissier, J. A. Morgan, N. Dudareva, ‘Plant volatiles: going ‘in’ but not ‘out’ of trichome cavities’, Trends Plant Sci. 2017, 22, 930–934.
- 9Z. Yosr, C. Hnia, T. Rim, B. Mohamed, ‘Changes in essential oil composition and phenolic fraction in Rosmarinus officinalis L. var. typicus Batt. organs during growth and incidence on the antioxidant activity’, Ind. Crops Prod. 2013, 43, 412–419.
- 10C. N. Hassiotis, F. Ntana, D. M. Lazari, S. Poulios, K. E. Vlachonasios, ‘Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period’, Ind. Crops Prod. 2014, 62, 359–366.
- 11M. Asadollahi-Baboli, A. Aghakhani, V. Bikdeloo, ‘Application of polyamide nanofibers, SPME/GC/MS, and chemometrics for comprehensive analysis of volatiles in Thymus vulgaris L. and Thymus serpyllum L’, Food Anal. Methods 2016, 9, 528–536.
- 12C. Crocoll, J. Asbach, J. Novak, J. Gershenzon, J. Degenhardt, ‘Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis’, Plant Mol. Biol. 2010, 73, 587–603.
- 13Z. A. Demissie, M. A. Cella, L. S. Sarker, T. J. Thompson, M. R. Rheault, S. S. Mahmoud, ‘Cloning, functional characterization and genomic organization of 1,8-cineole synthases from Lavandula’, Plant Mol. Biol. 2012, 79, 393–411.
- 14M. G. Miguel, F. Duarte, F. Venâncio, R. Tavares, ‘Variation in the main components of the essential oils from the leaves and flowers of Portuguese Thymus albicans over a single season’, J. Essent. Oil Res. 2004, 16, 169–171.
- 15R. C. S. Sá, L. N. Andrade, D. P. Sousa, ‘A review on anti-inflammatory activity of monoterpenes’, Molecules 2013, 18, 1227–1254.
- 16G. F. R. Caldas, A. R. da Silva Oliveira, A. V. Araújo, S. S. L. Lafayette, G. S. Albuquerque, J. C. Silva-Neto, J. H. Costa-Silva, F. Ferreira, J. G. M. da Costa, A. G. Wanderley, ‘Gastroprotective mechanisms of the monoterpene 1,8-cineole (Eucalyptol)’, PLoS One 2015, 10, e0134558.
- 17M. G. Miguel, A. C. Figueiredo, M. M. Costa, D. Martins, J. Duarte, J. G. Barroso, L. G. Pedro, ‘Effect of the volatile constituents isolated from Thymus albicans, T. mastichina T. carnosus and Thymbra capitata in sunflower oil’, Food / Nahrung 2003, 47, 397–402.
- 18A. Filipe, J. C. R. Cardoso, M. G. Miguel, L. Anjos, H. Trindade, A. C. Figueiredo, J. Barroso, D. M. Power, N. T. Marques, ‘Molecular cloning and functional characterization of a monoterpene synthase isolated from the aromatic wild shrub Thymus albicans’, J. Plant Physiol. 2017, 218, 35–44.
- 19A. C. Figueiredo, J. G. Barroso, L. G. Pedro, L. Salgueiro, M. G. Miguel, M. L. Faleiro, ‘Portuguese Thymbra and Thymus species volatiles: chemical composition and biological activities’, Curr. Pharm. Des. 2008, 14, 3120–3140.
- 20J. A. T. Silva, A. C. Figueiredo, J. G. Barroso, L. G. Pedro, L. Ascensão, ‘Estudo dos tricomas glandulares e do óleo essencial de Thymus mastichina L.’, Actas do 1° Colóquio Nacional de Plantas Aromáticas e Medicinais, Associação Portuguesa de Horticultura 1996, p. 229–232 (in Portuguese).
- 21X. Yu, C. Liang, H. Fang, X. Qi, W. Li, Q. Shang, ‘Variation of trichome morphology and essential oil composition of seven Mentha species’, Biochem. Syst. Ecol. 2018, 79, 30–36.
- 22L. B. Pérez-Estrada, Z. Cano-Santana, K. Oyama, ‘Variation in leaf trichomes of Wigandia urens: environmental factors and physiological consequences’, Tree Physiol. 2000, 20, 629–632.
- 23A. C. Figueiredo, J. G. Barroso, in ‘Medicinal and Aromatic Plants (MAP): How do they adapt to the environment?’, Ed. Á. Máthé, Springer, Dordrecht, 2015, p. 87.
- 24M. A. A. Souza, L. A. Santos, D. M. C. Brito, J. F. Rocha, R. N. Castro, M. S. Fernandes, S. R. Souza, ‘Influence of light intensity on glandular trichome density, gene expression and essential oil of menthol mint (Mentha arvensis L.)’, J. Essent. Oil Res. 2016, 28, 138–145.
- 25L. R. S. Tozin, M. M. O. Marques, T. M. Rodrigues, ‘Glandular trichome density and essential oil composition in leaves and inflorescences of Lippia origanoides Kunth (Verbenaceae) in the Brazilian Cerrado’, An. Acad. Bras. Cienc. 2015, 87, 943–953.
- 26S. Song, M. Gu, F. Chen, ‘Analysis on volatile components of flowers and leaves of Thymus mongolicus by SPME-GC/MS’, Med. Plant 2018, 9, 8–10.
- 27R. Rios-Estepa, I. Lange, J. M. Lee, B. M. Lange, ‘Mathematical modeling-guided evaluation of biochemical, developmental, environmental, and genotypic determinants of essential oil composition and yield in peppermint leaves’, Plant Physiol. 2010, 152, 2105–2119.
- 28G. W. Turner, E. M. Davis, R. B. Croteau, ‘Immunocytochemical localization of short-chain family reductases involved in menthol biosynthesis in peppermint’, Planta 2012, 235, 1185–1195.
- 29C. Liu, N. Srividya, A. N. Parrish, W. Yue, M. Shan, Q. Wu, B. M. Lange, ‘Morphology of glandular trichomes of Japanese catnip (Schizonepeta tenuifolia Briquet) and developmental dynamics of their secretory activity’, Phytochemistry 2018, 150, 23–30.
- 30A. Fähnrich, A. Brosemann, L. Teske, M. Neumann, B. Piechulla, ‘Synthesis of ‘cineole cassette’ monoterpenes in Nicotiana section Alatae: gene isolation, expression, functional characterization and phylogenetic analysis’, Plant Mol. Biol. 2012, 79, 537–553.
- 31M. L. Wise, T. J. Savage, E. Katahira, R. Croteau, ‘Monoterpene synthases from common sage (Salvia officinalis)’, J. Biol. Chem. 1998, 273, 14891–14899.
- 32A. Lane, A. Boecklemann, G. Woronuk, L. Sarker, S. Mahmoud, ‘A genomics resource for investigating regulation of essential oil production in Lavandula angustifolia’, Planta 2010, 231, 835–845.
- 33A. C. Figueiredo, M. S. Pais, ‘Ultrastructural aspects of the glandular cells from the secretory trichomes and from the cell suspension cultures of Achillea millefolium L. ssp. Millefolium’, Ann. Bot. 1994, 74, 179–190.
- 34C. A. Schneider, W. S. Rasband, K. W. Eliceiri, ‘NIH Image to ImageJ: 25 years of image analysis’, Nat. Methods 2012, 9, 671–675.
- 35A. M. Afonso, R. Guerra, A. M. Cavaco, P. Pinto, A. Andrade, A. Duarte, D. M. Power, N. T. Marques, ‘Identification of asymptomatic plants infected with Citrus tristeza virus from a time series of leaf spectral characteristics’, Comput. Electron. Agric. 2017, 141, 340–350.