Molecular Design, Synthesis and Docking Study of Alkyl and Benzyl Derivatives of Robustic Acid as Topoisomerase I Inhibitors
Rui Chen
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Faculty of Chinese Medicine Science, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJiayong Huang
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYogini Jaiswal
Center for Excellence in Post-Harvest Technologies, North Carolina A&T State University, The North Carolina Research Campus, 500 Laureate Way, Kannapolis, NC-28081 USA
Search for more papers by this authorJianhua Wei
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorCorresponding Author
Lini Huo
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorXing Xia
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorJing Zhong
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorLeonard Williams
Center for Excellence in Post-Harvest Technologies, North Carolina A&T State University, The North Carolina Research Campus, 500 Laureate Way, Kannapolis, NC-28081 USA
Search for more papers by this authorMaochun Huang
Faculty of Chinese Medicine Science, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorYan Liang
Guangxi Medical University, Nanning, 530021 P. R. China
Search for more papers by this authorRui Chen
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Faculty of Chinese Medicine Science, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJiayong Huang
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYogini Jaiswal
Center for Excellence in Post-Harvest Technologies, North Carolina A&T State University, The North Carolina Research Campus, 500 Laureate Way, Kannapolis, NC-28081 USA
Search for more papers by this authorJianhua Wei
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorCorresponding Author
Lini Huo
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorXing Xia
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorJing Zhong
Institute of Pharmacy, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorLeonard Williams
Center for Excellence in Post-Harvest Technologies, North Carolina A&T State University, The North Carolina Research Campus, 500 Laureate Way, Kannapolis, NC-28081 USA
Search for more papers by this authorMaochun Huang
Faculty of Chinese Medicine Science, Guangxi University of Chinese Medicine, Nanning, 530222 P. R. China
Search for more papers by this authorYan Liang
Guangxi Medical University, Nanning, 530021 P. R. China
Search for more papers by this authorAbstract
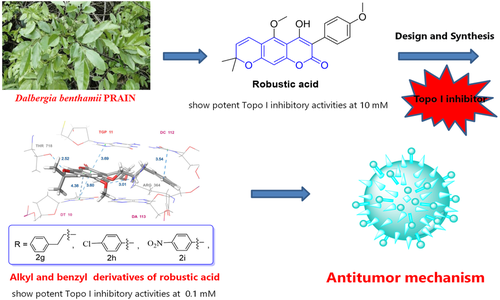
Robustic acid is reported to be a bioactive compound, isolated from the medicinal plant Dalbergia benthamii Prain. Ten alkyl and benzyl derivatives (2a–2j) of robustic acid were designed and synthesized based on molecular docking approaches. The biological activities of most of the synthesized compounds (such as 2g, 2h, and 2i) were closely consistent with the docking results. In particular, 4-O-phenylpropyl substituted compound 2g displayed potent topoisomerase I inhibitory activity as well as cytotoxicity against SMMC-7721, HepG2, and HeLa cell lines. Further biological testing suggests that compound 2g acted mainly by an arrest of the tumor cells in G1 phase of the cell cycle and suppressed cell proliferation by inducing apoptosis. The findings of this study are encouraging with respect to potential utilization of these compounds as new topoisomerase I inhibitors.
Graphical Abstract
References
- 1L. Huang, Z. L. Feng, Y. T. Wang, L. G. Lin, ‘Anticancer Carbazole Alkaloids and Coumarins from Clausena Plants: A Review’, Chin. J. Nat. Med. 2017, 15, 881–888.
- 2A. Guruge, C. S. Udawatte, S. Weerasinghe, ‘An in Silico Approach of Coumarin Derived Inhibitors for Human DNA Topoisomerase I’, Aust. J. Chem. 2016, 69, 1005–1015.
- 3L. H. Meng, Z. Y. Liao, Y. Pommier, ‘Non-Camptothecin DNA Topoisomerase I Inhibitors in Cancer Therapy’, Curr. Top. Med. Chem. 2003, 3, 305–320.
- 4N. S. Reddy, M. S. Mallireddigari, K. Gumireddy, S. C. Bell, E. P. Reddy, M. V. Reddy, ‘Synthesis of New Coumarin 3-(N-Aryl) Sulfonamides and Their Anticancer Activity’, Bioorg. Med. Chem. Lett. 2004, 14, 4093–4097.
- 5T. Devji, C. Reddy, C. Woo, S. Awale, S. Kadota, D. Carrico-Moniz, ‘Pancreatic Anticancer Activity of a Novel Geranylgeranylated Coumarin Derivative’, Bioorg. Med. Chem. Lett. 2011, 21, 5770–5773.
- 6J. Klenkar, M. Molnar, ‘Natural and Synthetic Coumarins as Potential Anticancer Agents’, J. Chem. Pharm. Res. 2015, 7, 1223–1238.
- 7A. Thakur, R. Singla, V. Jaitak, ‘Coumarins as Anticancer Agents: A Review on Synthetic Strategies, Mechanism of Action and SAR Studies’, Eur. J. Med. Chem. 2015, 101, 476–495.
- 8D. Kumar, P. Sharma, H. Singh, K. Nepali, F. Ntie-Kang, ‘The Value of Pyrans as Anticancer Scaffolds in Medicinal Chemistry’, RSC Adv. 2017, 7, 36977–36999.
- 9C. R. Su, S. F. Yeh, C. M. Liu, A. G. Damu, T. H. Kuo, P. C. Chiang, K. F. Bastow, K. H. Lee, T. S. Wu, ‘Anti-Hbv and Cytotoxic Activities of Pyranocoumarin Derivatives’, Bioorg. Med. Chem. 2009, 17, 6137–6143.
- 10J. Cao, S. Shen, P. Yang, J. Qu, ‘A Catalyst-Free One-Pot Construction of Skeletons of 5-Methoxyseselin and Alloxanthoxyletin in Water’, Org. Lett. 2013, 15, 3856–3859.
- 11W. W. Peng, G. Z. Zeng, W. W. Song, N. H. Tan, ‘A New Cytotoxic Carbazole Alkaloid and Two New Other Alkaloids from Clausena Excavata’, Chem. Biodiversity 2013, 10, 1317–1321.
- 12J. H. Wei, L. N. Huo, M. Huang, R. Chen, X. Feng, ‘Robustic Acid and Its Extraction Method and Application’, 2014, CN201410522224.9 (patent).
- 13R. Chen, L. N. Huo, Y. Jaiswal, J. H. Wei, D. P. Li, J. Zhong, L. Williams, X. Xia, Y. Liang, ‘Synthesis and Evaluation of Anticancer Activity of New 4-Acyloxy Derivatives of Robustic Acid’, Int. J. Mol. Sci. 2019, 20, 5336; doi:10.3390/ijms20215336.
- 14M. Rangarajan, J. S. Kim, S. P. Sim, A. Liu, L. F. Liu, E. J. Lavoie, ‘Topoisomerase I Inhibition and Cytotoxicity of 5-Bromo- and 5-Phenylterbenzimidazoles’, Bioorg. Med. Chem. 2000, 8, 2591–2600.
- 15M. M. F. Ismaila, M. M. M. Hussein, ‘Synthesis and Docking Studies of Novel Benzopyran-2-ones with Anticancer Activity’, Eur. J. Med. Chem. 2010, 45, 3950–3959.
- 16L. X. Zhao, T. S. Kim, S.-H. Ahn, T.-H. Kim, E.-K. Kim, W.-J. Cho, H. Choi, C.-S. Lee, J.-A. Kim, T. C. Jeong, C.-J. Chang, E.-S. Lee, ‘Synthesis, Topoisomerase I Inhibition and Antitumor Cytotoxicity of 2,2′ : 6′,2′′-, 2,2′ : 6′,3′′- and 2,2′ : 6′,4′′-Terpyridine Derivatives’, Bioorg. Med. Chem. Lett. 2001, 11, 2659–2662.
- 17L. Kai, D. D. Li, X. M. Zhao, L. L. Dai, T. Zhang, Z. W. Tao, ‘Synthesis, Cytotoxicity, Topoisomerase I Inhibition and Molecular Docking of Novel Phosphoramide Mustard Sophoridinic Acid Analogs: Phosphoramide Mustard Sophoridinic Acid Analogs as Topo I Inhibitors’, Appl. Organomet. Chem. 2017, 31, 1–7.
- 18Z. Yuan, S. Chen, C. Chen, J. Chen, C. Chen, Q. Dai, C. Gao, Y. Jiang, ‘Design, Synthesis and Biological Evaluation of 4-Amidobenzimidazole Acridine Derivatives as Dual Parp and Topo Inhibitors for Cancer Therapy’, Eur. J. Med. Chem. 2017, 138, 1135–1146.
- 19D. M. X. Donnelly, D. J. Molloy, J. P. Reilly, J. Finet, ‘Aryllead-mediated synthesis of linear 3-arylpyranocoumarins: synthesis of robustin and robustic acid’, J. Chem. Soc., Perkin Trans. 1 1995, 20, 2531–2534.
10.1039/p19950002531 Google Scholar
- 20S. A. Khalid, P. G. Waterman, ‘Thonningin A and Thonningin B: two 3-phenylcoumarins from the seeds of Millettia thonningii’, Phytochemistry 1983, 22, 1001–1003.
- 21X. C. Huang, M. Wang, H. S. Wang, Z. F. Chen, Y. Zhang, Y. M. Pan, ‘Synthesis and Antitumor Activities of Novel Dipeptide Derivatives Derived from Dehydroabietic Acid’, Bioorg. Med. Chem. Lett. 2014, 24, 1511–1518.
- 22M. V. Berridge, A. S. Tan, ‘Characterization of the Cellular Reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction’, Arch. Biochem. Biophys. 1993, 303, 474–482.
- 23W. J. Cho, E. K. Kim, I. Y. Park, E. Y. Jeong, T. S. Kim, T. N. Le, D. D. Kim, E. S. Lee, ‘Molecular Modeling of 3-Arylisoquinoline Antitumor Agents Active Against A-549. A Comparative Molecular Field Analysis Study’, Bioorg. Med. Chem. 2002, 10, 2953–2961.
- 24K. E. Hevener, W. Zhao, R. E. Lee, ‘Validation of Molecular Docking Programs for Virtual Screening against Dihydropteroate Synthase’, J. Chem. Inf. Model. 2009, 49, 444–460.
- 25M. Lill, ‘Virtual Screening in Drug Design’, In Silico Models Drug Discov. 2013, 993, 1–12.