Novel N-Acyl-1H-imidazole-1-carbothioamides: Design, Synthesis, Biological and Computational Studies
Hamid Aziz
Department of Chemistry, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorCorresponding Author
Aamer Saeed
Department of Chemistry, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorMuhammad Aslam Khan
Department of Biotechnology, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorShakeeb Afridi
Department of Biotechnology, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorFarukh Jabeen
Department of Biology, Laurentian University, 935 Ramsey Lake Road, Sudbury, ON, Canada, P3E 2C6
Computation, Science, Research and Development Organization, 1401, 2485 Hurontraio Street, Mississauga, ON, Canada, L5A 2G6
Search for more papers by this authorAshfaq-ur-Rehman
Department of Biochemistry, Shankar Campus, Abdul Wali Khan University, Mardan, 23200, Khyber Pukhtoonkhwa Pakistan
Department of Bioinformatics and Biostatistics, Shanghai Jiao Tong University, 800 Dongchuan Road, Minhang District Shanghai, Shanghai, 200240 P. R. China
Search for more papers by this authorMuhammad Hashim
Department of Biochemistry, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorHamid Aziz
Department of Chemistry, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorCorresponding Author
Aamer Saeed
Department of Chemistry, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorMuhammad Aslam Khan
Department of Biotechnology, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorShakeeb Afridi
Department of Biotechnology, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorFarukh Jabeen
Department of Biology, Laurentian University, 935 Ramsey Lake Road, Sudbury, ON, Canada, P3E 2C6
Computation, Science, Research and Development Organization, 1401, 2485 Hurontraio Street, Mississauga, ON, Canada, L5A 2G6
Search for more papers by this authorAshfaq-ur-Rehman
Department of Biochemistry, Shankar Campus, Abdul Wali Khan University, Mardan, 23200, Khyber Pukhtoonkhwa Pakistan
Department of Bioinformatics and Biostatistics, Shanghai Jiao Tong University, 800 Dongchuan Road, Minhang District Shanghai, Shanghai, 200240 P. R. China
Search for more papers by this authorMuhammad Hashim
Department of Biochemistry, Quaid-I-Azam University, Islamabad, 45320 Pakistan
Search for more papers by this authorAbstract
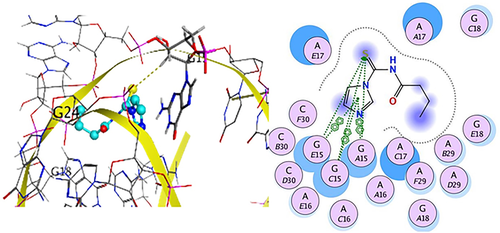
The present study reports the convenient synthesis, spectroscopic characterization, bio-assays and computational evaluation of a novel series of N-acyl-1H-imidazole-1-carbothioamides. The screened derivatives displayed excellent antioxidant activity, moderate antibacterial and antifungal potential. The screened derivatives were found to be highly biocompatible against hRBCs. Molecular docking ascertained the mechanism and mode of action towards the molecular target delineating that ligands and complexes were stabilized at the active site by electrostatic and hydrophobic forces in accordance to the corresponding experimental results. Docking simulation provided additional information about the possibilities of inhibitory potential of the compounds against RNA. Computational evaluation predicted that N-acyl-1H-imidazole-1-carbothioamides 5c and 5g can serve as potential surrogates for hit to lead generation and design of novel antioxidant and antibacterial agents.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv201900509-sup-0001-misc_information.pdf916.5 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. Birben, U. M. Sahiner, C. Sackesen, S. Erzurum, O. Kalayci, ‘Oxidative stress and antioxidant defense’, World Allergy Organ. J. 2012, 5, 9–19.
- 2S. Süzen, ‘Antioxidant activities of synthetic indole derivatives and possible activity mechanisms’, Bioact. Heterocyl. V 2007, 11, 145–178.
10.1007/7081_2007_074 Google Scholar
- 3M. Valko, C. J. Rhodes, J. Moncol, M. Izakovic, M. Mazur, ‘Free radicals, metals and antioxidants in oxidative stress-induced cancer’, Chem.-Biol. Interact. 2006, 160, 1–40.
- 4Y. Y. Liu, Y. Wang, T. R. Walsh, L. X. Yi, R. Zhang, J. Spencer, Y. Doi, G. Tian, B. Dong, X. Huang, L. F. Yu, ‘Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study’, Lancet Infect. Dis. 2016, 16, 161–168.
- 5M. K. Rai, S. D. Deshmukh, A. P. Ingle, A. K. Gade, ‘Silver nanoparticles: the powerful nano weapon against multidrug-resistant bacteria’, J. Appl. Microbiol. 2012, 112, 841–852.
- 6R. J. Worthington, C. Melander, ‘Combination approaches to combat multidrug-resistant bacteria’, Trends Biotechnol. 2013, 31, 177–184.
- 7L. Dewachter, M. Fauvart, J. Michiels, ‘Bacterial Heterogeneity and Antibiotic Survival: Understanding and Combatting Persistence and Heteroresistance’, Mol. Cell 2019, 76, 255–267.
- 8Y. Dong, T. K. Venkatachalam, R. K. Narla, V. N. Trieu, E. A. Sudbeck, F. M. Uckun, ‘Antioxidant function of phenethyl-5-bromo-pyridylthiourea compounds with potent anti-HIV activity’, Bioorg. Med. Chem. Lett. 2000, 10, 87–90.
- 9A. P. Keche, G. D. Hatnapure, R. H. Tale, A. H. Rodge, S. S. Birajdar, V. M. Kamble, ‘A novel pyrimidine derivative with aryl urea, thiourea and sulfonamide moieties: synthesis, anti-inflammatory and antimicrobial evaluation’, Bioorg. Med. Chem. Lett. 2012, 22, 3445–3448.
- 10A. Saeed, U. Shaheen, A. Hameed, S. H. Naqvi, ‘Synthesis, characterization and antimicrobial activity of some new 1-(fluorobenzoyl)-3-(fluorophenyl)thioureas’, J. Fluorine Chem. 2009, 130, 1028–1034.
- 11A. Saeed, U. Flörke, M. F. Erben, ‘A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas’, J. Sulfur. Chem. 2014, 35, 318–355.
- 12A. Saeed, R. Qamar, T. A. Fattah, U. Florke, M. F. Erben, ‘Recent developments in chemistry, coordination, structure and biological aspects of 1-(acyl/aroyl)-3-(substituted) thioureas’, Res. Chem. Intermed. 2017, 43, 3053–3093.
- 13S. Madabhushi, K. K. R. Mallu, V. S. Vangipuram, S. Kurva, Y. Poornachandra, C. G. Kumar, ‘Synthesis of novel benzimidazole functionalized chiral thioureas and evaluation of their antibacterial and anticancer activities’, Bioorg. Med. Chem. Lett. 2014, 24, 4822–4825.
- 14K. Ekoue-Kovi, K. Yearick, D. P. Iwaniuk, J. K. Natarajan, J. Alumasa, A. C. de Dios, C. Wolf, ‘Synthesis and antimalarial activity of new 4-amino-7-chloroquinolyl amides, sulfonamides, ureas and thioureas’, Bioorg. Med. Chem. 2009, 17, 270–283.
- 15D. Sriram, P. Yogeeswari, K. Madhu, ‘Synthesis and in vitro antitubercular activity of some 1-[(4-sub)phenyl]-3-(4-{1-[(pyridine-4-carbonyl)hydrazono]ethyl}phenyl) thiourea’, Bioorg. Med. Chem. Lett. 2006, 16, 876–878.
- 16T. K. Venkatachalam, C. Mao, F. M. Uckun, ‘Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds’, Bioorg. Med. Chem. 2004, 12, 4275–4284.
- 17J. Lee, J. Kim, S. Y. Kim, M. W. Chun, H. Cho, M. Beheshti, ‘N-(3-Acyloxy-2-benzylpropyl)-N′-(4-hydroxy-3-methoxybenzyl)thiourea derivatives as potent vanilloid receptor agonists and analgesics’, Bioorg. Med. Chem. 2001, 9, 19–32.
- 18R. S. Viswas, S. Pundir, H. Lee, ‘Design and synthesis of 4-piperazinyl quinoline derived urea/thioureas for anti-breast cancer activity by a hybrid pharmacophore approach’, J. Enzyme. Inhib. Med. Chem. 2019, 34, 620–630.
- 19R. Gondru, S. R. Peddi, V. Manga, M. Khanapur, R. Gali, N. Sirassu, R. Bavantula, ‘One-pot synthesis, biological evaluation and molecular docking studies of fused thiazolo[2,3-b]pyrimidinone-pyrazolylcoumarin hybrids’, Mol. Diversity 2018, 22, 943–956.
- 20Y. K. Gupta, V. Gupta, S. Singh, ‘Synthesis, characterization and antimicrobial activity of pyrimidine based derivatives’, J. Pharm. Res. 2013, 7, 491–495.
- 21S. Saeed, N. Rashid, P. G. Jones, M. Ali, R. Hussain, ‘Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents’, Eur. J. Med. Chem. 2010, 4, 1323–1331.
- 22E. C. Aguilar, G. A. Echeverría, O. E. Piro, S. E. Ulic, J. L. Jios, M. E. Tuttolomondo, M. E. Arena, ‘Acyl thiourea derivatives: A study of crystallographic, bonding, biological and spectral properties’, Chem. Phys. Lett. 2019, 715, 64–71.
- 23M. Chandrasekhar, G. S. Prasad, C. Venkataramaiah, C. N. Raju, K. Seshaiah, W. Rajendra, ‘Synthesis, spectral characterization, docking studies and biological activity of urea, thiourea, sulfonamide and carbamate derivatives of imatinib intermediate’, Mol. Diversity 2018, 23, 1–16.
- 24Y. Qin, W. Liu, R. Xing, S. Liu, K. Li, P. Li, ‘Cyclization Reaction of Acyl Thiourea Chitosan: Enhanced Antifungal Properties via Structural Optimization’, Molecules 2018, 23, 594.
- 25Z. Guo, X. Song, L. M. Zhao, M. G. Piao, J. Quan, H. R. Piao, C. H. Jin, ‘Synthesis and biological evaluation of novel benzo[c][1,2,5]thiadiazol-5-yl and thieno[3,2-c]pyridin-2-yl imidazole derivatives as ALK5 inhibitors’, Bioorg. Med. Chem. Lett. 2019, 29, 2070–2075.
- 26W. Brand-Williams, M. E. Cuvelier, C. L. W. T. Berset, ‘Use of a free radical method to evaluate antioxidant activity’, LWT – Food Sci. Technol. 1995, 28, 25–30.
- 27M. Umamaheswari, T. K. Chatterjee, ‘In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract’, Afr. J. Tradit. Complementary Altern. Med. 2008, 5, 61–73.
- 28P. Siddhuraju, P. S. Mohan, K. Becker, ‘Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp’, Food Chem. 2002, 79, 61–67.
- 29B. C. Evans, C. E. Nelson, S. Y. Shann, K. R. Beavers, A. J. Kim, H. Li, C. L. Duvall, ‘Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs’, J. Vis. Exp. 2013, 73, e50166.
- 30K. Amin, R. M. Dannenfelser, ‘In vitro hemolysis: guidance for the pharmaceutical scientist’, J. Pharm. Sci. 2006, 95, 1173–1176.
- 31Q. Vicens, E. Westhof, ‘Crystal structure of paromomycin docked into the eubacterial ribosomal decoding A site’, Structure 2001, 9, 647–658.
- 32A. U. Rehman, H. Rafiq, M. U. Rahman, J. Li, H. Liu, S. Luo, T. Arshad, A. Wadood, H. F. Che, ‘Gain-of-Function SHP2 E76Q Mutant Rescuing Autoinhibition Mechanism Associated with Juvenile Myelomonocytic Leukemia’, J. Chem. Inf. Model. 2019, 59, 3229–3239.
- 33A. U. Rehman, M. T. Khan, H. Liu, A. Wadood, S. I. Malik, H. F. Chen, ‘Exploring the Pyrazinamide Drug Resistance Mechanism of Clinical Mutants T370P and W403G in Ribosomal Protein S1 of Mycobacterium tuberculosis’, J. Chem. Inf. Model. 2019, 59, 1584–1597.
- 34C. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, ‘Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings’, Adv. Drug Delivery Rev. 1997, 23, 3–25.
- 35D. F. Veber, S. R. Johnson, H. Y. Cheng, B. R. Smith, K. W. Ward, K. D. Kopple, ‘Molecular properties that influence the oral bioavailability of drug candidates’, J. Med. Chem. 2002, 45, 2615–2623.
- 36A. Saeed, A. Khurshid, M. Bolte, A. C. Fantoni, M. F. Erben, ‘Intra- and intermolecular hydrogen bonding and conformation in 1-acyl thioureas: An experimental and theoretical approach on 1-(2-chlorobenzoyl)thiourea’, Spectrochim. Acta Part A 2015, 143, 59–66.
- 37S. S. Zahra, M. Ahmed, M. Qasim, B. Gul, M. Zia, B. Mirza, I. U. Haq, ‘Polarity based characterization of biologically active extracts of AJR Am. J. Roentgenol. Wall. ex Benth. and RP-HPLC analysis’, BMC Complementary Altern. Med. 2017, 17, 443.
- 38M. Umamaheswari, T. K. Chatterjee, ‘The effect of natural antioxidants extracted from plant and animal resources on the oxidative stability of soybean oil’, Afr. J. Tradit. Complementary Altern. Med. 2008, 5, 61–73.
- 39M. Taghvaei, S. M. Jafari, A. S. Mahoonak, A. M. Nikoo, N. Rahmanian, J. Hajitabar, N. Meshginfar, ‘The effect of natural antioxidants extracted from plant and animal resources on the oxidative stability of soybean oil’, LWT - Food Sci. Technol. 2014, 56, 124–130.
- 40M. Jamil, I. Haq, B. Mirza, M. Qayyum, ‘Isolation of antibacterial compounds from Quercus dilatata L. through bioassay guided fractionation’, Ann. Clin. Microbiol. Antimicrob. 2012, 11, 11.
- 41M. Ahmed, H. Fatima, M. Qasim, B. Gul, ‘Polarity directed optimization of phytochemical and in vitro biological potential of an indigenous folklore: Quercus dilatata Lindl. ex Royle’, BMC Complementary Altern. Med. 2017, 17, 386.
- 42Molecular Operating Environment, 20118.01, Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite 910, Montreal, QC, Canada, H3A 2R7, 2018.
- 43T. A. Halgren, R. B. Nachbar, ‘Merck molecular force field. IV. Conformational energies and geometries for MMFF94’, J. Comput. Chem. 1996, 17, 587–615.
- 44G. Menozzi, L. Merello, P. Fossa, S. Schenone, A. Ranise, L. Mosti, P. La Colla, ‘Synthesis, antimicrobial activity and molecular modeling studies of halogenated 4-[1H-imidazol-1-yl (phenyl) methyl]-1,5-diphenyl-1H-pyrazoles’, Bioorg. Med. Chem. 2004, 12, 5465–5483.
- 45J. Wang, W. Wang, P. A. Kollman, D. A. Case, ‘Automatic atom type and bond type perception in molecular mechanical calculations’, J. Mol. Graphics Modell. 2006, 25, 247–260.
- 46D. A. Case, I. Y. Ben-Shalom, S. R. Brozell, D. S. Ceritti, T. E. Cheatham III, V. W. D. Cruzeiro, T. A. Darden, R. E. Duke, D. Ghoreishi, M. K. Gilson, H. Gohlke, A. W. Goetz, D. Greene, R. Harris, N. H. Homeyer, S. Izadi, A. Kovalenko, T. Kurtzman, T. S. Lee, S. LeGrand, P. Li, C. Lin, J. Liu, T. Luchko, R. Luo, D. J. Mermelstein, K. M. Merz, Y. Miao, G. Monard, C. Nguyen, H. Nguyen, I. Omelyan, A. Onufriev, F. Pan, R. Qi, D. R. Roe, A. Roitberg, C. Sagui, S. Schot-Verdugo, J. Shen, C. L. Simmerling, J. Smith, R. Salomon-Ferrer, J. Swails, R. C. Walker, J. Wang, H. Wei, R. M. Wolf, X. Wu, L. Xiao, D. M. York, P. A. Kollman, ‘AMBER 2018: University of California San Francisco’, 2018.
- 47T. Y. Darden, D. P. Lee, ‘Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems’, J. Chem. Phys. 1993, 98, 10089–10092.
- 48X. Wu, B. R. Brooks, E. Vanden-Eijnden, ‘Self-guided Langevin dynamics via generalized Langevin equation’, J. Comput. Chem. 2016, 37, 595–601.