Organocatalyzed Solvent Free and Efficient Synthesis of 2,4,5-Trisubstituted Imidazoles as Potential Acetylcholinesterase Inhibitors for Alzheimer's Disease
Sania Pervaiz
Department of Chemistry, Government College, University, Lahore, 54000 Pakistan
Search for more papers by this authorCorresponding Author
Sadaf Mutahir
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094 P. R. China
Search for more papers by this authorCorresponding Author
Islam Ullah
Department of Chemistry, Government College, University, Lahore, 54000 Pakistan
Department of Chemistry, Faculty of Sciences, University of Sialkot, Sialkot, 51040 Pakistan
Search for more papers by this authorMuhammad Ashraf
Department of Chemistry and Department of Biochemistry and Biotechnology, The Islamia University of Bahawalpur, Bahawalpur, 63100 Pakistan
Search for more papers by this authorXiao Liu
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094 P. R. China
Search for more papers by this authorSidrah Tariq
Department of Chemistry, Government College, University, Lahore, 54000 Pakistan
Search for more papers by this authorBao-Jing Zhou
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094 P. R. China
Search for more papers by this authorCorresponding Author
Muhammad Asim Khan
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094 P. R. China
Search for more papers by this authorSania Pervaiz
Department of Chemistry, Government College, University, Lahore, 54000 Pakistan
Search for more papers by this authorCorresponding Author
Sadaf Mutahir
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094 P. R. China
Search for more papers by this authorCorresponding Author
Islam Ullah
Department of Chemistry, Government College, University, Lahore, 54000 Pakistan
Department of Chemistry, Faculty of Sciences, University of Sialkot, Sialkot, 51040 Pakistan
Search for more papers by this authorMuhammad Ashraf
Department of Chemistry and Department of Biochemistry and Biotechnology, The Islamia University of Bahawalpur, Bahawalpur, 63100 Pakistan
Search for more papers by this authorXiao Liu
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094 P. R. China
Search for more papers by this authorSidrah Tariq
Department of Chemistry, Government College, University, Lahore, 54000 Pakistan
Search for more papers by this authorBao-Jing Zhou
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094 P. R. China
Search for more papers by this authorCorresponding Author
Muhammad Asim Khan
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094 P. R. China
Search for more papers by this authorAbstract
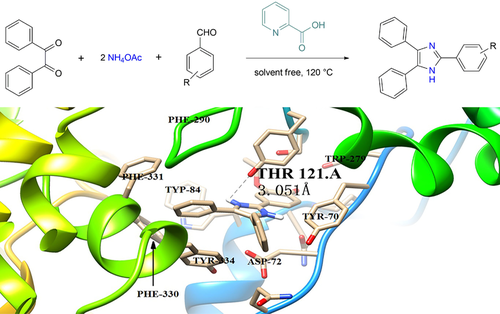
The catalytic potential of pyridine-2-carboxlic acid has been evaluated for efficient, green and solvent free synthesis of 2,4,5-trisubstituted imidazole derivatives 3a–3m. The compounds 3a–3m were synthesized by one pot condensation reaction of substituted aromatic aldehydes, benzil, and ammonium acetate in good to excellent yields (74–96 %). To explore the potential of these compounds against Alzheimer's disease, their inhibitory activities against acetylcholinesterase (AChE) were evaluated. In this series of compounds, compound 3m, bearing one ethoxy and a hydroxy group on the phenyl ring on 2,4,5-trisubstituted imidazoles, proved to be a potent AChE inhibitor (102.56±0.14). Structure–activity relationship (SAR) of these compounds was developed. Molecular dockings were carried out for the compounds 3m, 3e, 3k, 3c, 3a, 3d, 3j, and 3f in order to further investigate the binding mechanism. The inhibitor molecule was molecularly docked with acetylcholinesterase to further study its binding mechanism. The amino group of the compound 3m forms an H-bond with the oxygen atom of the residue (i. e., THR121) which has a bond length of 3.051 Å.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv201900493-sup-0001-misc_information.pdf1.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. J. Connon, ‘Asymmetric organocatalytic reductions mediated by dihydropyridines’, Org. Biomol. Chem. 2007, 21, 3407–3417.
- 2P. R. Schreiner, ‘Metal-free organocatalysis through explicit hydrogen bonding interactions’, Chem. Soc. Rev. 2003, 32, 289–296.
- 3D. W. C. MacMillan, ‘The advent and development of organocatalysis’, Nature 2008, 455, 304–308.
- 4I. R. Shaikh, ‘Organocatalysis: key trends in green synthetic chemistry, challenges, scope towards heterogenization, and importance from research and industrial point of view’, J. Catal. 2014, 2014.
- 5S. Mutahir, M. A. Khan, I. U. Khan, M. Yar, M. Ashraf, S. Tariq, R. L. Ye, B. J. Zhou, ‘Organocatalyzed and mechanochemical solvent-free synthesis of novel and functionalized bis-biphenyl substituted thiazolidinones as potent tyrosinase inhibitors: SAR and molecular modeling studies’, Eur. J. Med. Chem. 2017, 134, 406–414.
- 6M. Yar, M. Bajda, L. Shahzadi, S. A. Shahzad, M. Ahmed, M. Ashraf, U. Alam, I. U. Khan, A. F. Khan, ‘Novel synthesis of dihydropyrimidines for α-glucosidase inhibition to treat type 2 diabetes: In vitro biological evaluation and in silico docking’, Bioorg. Chem. 2014, 54, 96–104.
- 7R. Anand, K. D. Gill, A. A. Mahdi, ‘Therapeutics of Alzheimer's disease: Past, present and future’, Neuropharmacology 2014, 76, 27–50.
- 8C. P. Ferri, M. Prince, C. Brayne, H. Brodaty, L. Fratiglioni, M. Ganguli, K. Hall, K. Hasegawa, H. Hendrie, Y. Huang, A. Jorm, ‘Global prevalence of dementia: a Delphi consensus study’, Lancet 2005, 366, 2112–2117.
- 9J. Godyń, J. Jończyk, D. Panek, B. Malawska, ‘Therapeutic strategies for Alzheimer's disease in clinical trials’, Pharmacol. Rep. 2016, 68, 127–138.
- 10Y. Li, X. Qiang, Y. Li, X. Yang, L. Luo, G. Xiao, Z. Cao, Z. Tan, Y. Deng, ‘Pterostilbene-O-acetamidoalkylbenzylamines derivatives as novel dual inhibitors of cholinesterase with anti-β-amyloid aggregation and antioxidant properties for the treatment of Alzheimer's disease’, Bioorg. Med. Chem. Lett. 2016, 26, 2035–2039.
- 11P. Anand, B. Singh, N. Singh, ‘A review on coumarins as acetylcholinesterase inhibitors for Alzheimer's disease’, Bioorg. Med. Chem. 2012, 20, 1175–1180.
- 12H. Tang, F. X. Ning, Y. B. Wei, S. L. Huang, Z. S. Huang, A. S. C. Chan, L. Q. Gu, ‘Derivatives of oxoisoaporphine alkaloids: a novel class of selective acetylcholinesterase inhibitors’, Bioorg. Med. Chem. Lett. 2007, 17, 3765–3768.
- 13M. Saeedi, D. Mohtadi-Haghighi, S. S. Mirfazli, M. Mahdavi, R. Hariri, H. Lotfian, N. Edraki, A. Iraji, O. Firuzi, T. Akbarzadeh, ‘Design and Synthesis of Selective Acetylcholinesterase Inhibitors: Arylisoxazole-Phenylpiperazine Derivatives’, Chem. Biodiversity 2019, 16, e1800433.
- 14S. Mutahir, J. Jończyk, M. Bajda, I. U. Khan, M. A. Khan, N. Ullah, M. Ashraf, S. Riaz, S. Hussain, M. Yar, ‘Novel biphenyl bis-sulfonamides as acetyl and butyrylcholinesterase inhibitors: Synthesis, biological evaluation and molecular modeling studies’, Bioorg. Chem. 2016, 64, 13–20.
- 15T. U. Rehman, I. U. Khan, M. Ashraf, H. Tarazi, S. Riaz, M. Yar, ‘An Efficient Synthesis of Biaryl Pyrimidine Heterocycles: Potential New Drug Candidates to Treat Alzheimer's Disease’, Arch. Pharm. 2017, 350, e1600304.
- 16S. Riaz, I. U. Khan, M. Bajda, M. Ashraf, A. Shaukat, T. U. Rehman, S. Mutahir, S. Hussain, G. Mustafa, M. Yar, ‘Pyridine sulfonamide as a small key organic molecule for the potential treatment of type-II diabetes mellitus and Alzheimer's disease: in vitro studies against yeast α-glucosidase, acetylcholinesterase and butyrylcholinesterase’, Bioorg. Chem. 2015, 63, 64–71.
- 17N. H. Lin, L. Wang, J. Cohen, W. Z. Gu, D. Frost, H. Zhang, S. Rosenberg, H. Sham, ‘Synthesis and biological evaluation of 4-[3-biphenyl-2-yl-1-hydroxy-1-(3-methyl-3H-imidazol-4-yl)-prop-2-ynyl]-1-yl-benzonitrile as novel farnesyltransferase inhibitor’, Bioorg. Med. Chem. Lett. 2003. 13, 1293–1296.
- 18D. Niculescu-Duvaz, I. Niculescu-Duvaz, B. M. Suijkerbuijk, D. Ménard, A. Zambon, L. Davies, J. F. Pons, S. Whittaker, R. Marais, C. J. Springer, ‘Potent BRAF kinase inhibitors based on 2,4,5-trisubstituted imidazole with naphthyl and benzothiophene 4-substituents’, Bioorg. Med. Chem. 2013, 21, 1284–1304.
- 19T. Mano, R. W. Stevens, K. Ando, K. Nakao, Y. Okumura, M. Sakakibara, T. Okumura, T. Tamura, K. Miyamoto, ‘Novel imidazole compounds as a new series of potent, orally active inhibitors of 5-lipoxygenase’, Bioorg. Med. Chem. 2003, 11, 3879–3887.
- 20T. F. Gallagher, G. L. Seibel, S. Kassis, J. T. Laydon, M. J. Blumenthal, J. C. Lee, D. Lee, J. C. Boehm, S. M. Fier-Thompson, J. W. Abt, M. E. Soreson, ‘Regulation of stress-induced cytokine production by pyridinylimidazoles; inhibition of CSBP kinase’, Bioorg. Med. Chem. 1997, 5, 49–64.
- 21J. C. Lee, A. M. Badger, D. E. Griswold, D. Dunnington, A. Truneh, B. Votta, J. R. White, P. R. Young, P. E. Bender, ‘Bicyclic imidazoles as a novel class of cytokine biosynthesis inhibitors’, Ann. N. Y. Acad. Sci. 1993, 696, 149–170.
- 22I. K. Khanna, R. M. Weier, Y. Yu, X. D. Xu, F. J. Koszyk, P. W. Collins, C. M. Koboldt, A. W. Veenhuizen, W. E. Perkins, J. J. Casler, J. L. Masferrer, ‘1,2-Diarylimidazoles as potent, cyclooxygenase-2 selective, and orally active anti-inflammatory agents’, J. Med. Chem. 1997, 40, 1634–1647.
- 23M. Esmaeilpour, J. Javidi, M. Zandi, ‘One-pot synthesis of multisubstituted imidazoles under solvent-free conditions and microwave irradiation using Fe3O4@SiO2-imid-PMAn magnetic porous nanospheres as a recyclable catalyst’, New J. Chem. 2015, 3388–3398.
- 24A. Shaabani, A. Rahmati, ‘Silica sulfuric acid as an efficient and recoverable catalyst for the synthesis of trisubstituted imidazoles’, J. Mol. Catal. A: Chem. 2006, 249, 246–248.
- 25M. Kidwai, P. Mothsra, V. Bansal, R. K. Somvanshi, A. S. Ethayathulla, S. Dey, S. T. P. Singh, ‘One-pot synthesis of highly substituted imidazoles using molecular iodine: A versatile catalyst’, J. Mol. Catal. A: Chem. 2007, 265, 177–182.
- 26B. Sadeghi, B. B. F. Mirjalili, M. M. Hashemi, ‘BF3⋅SiO2: an efficient reagent system for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles’, Tetrahedron Lett. 2008, 49, 2575–2577.
- 27A. R. Khosropour, ‘Ultrasound-promoted greener synthesis of 2,4,5-trisubstituted imidazoles catalyzed by Zr(acac)4 under ambient condition’, Ultrason. Sonochem. 2008, 15, 659–664.
- 28M. Kidwai, P. Mothsra, V. Bansal, R. K. Somvanshi, A. S. Ethayathulla, S. Dey, T. P. Singh, ‘Scolecite as an efficient heterogeneous catalyst for the synthesis of 2,4,5-triarylimidazoles’, Open Chem. 2009, 7, 550–554.
- 29J. Safari, S. D. Khalili, M. Rezaei, S. H. Banitaba, F. Meshkani, ‘Nanocrystalline magnesium oxide: a novel and efficient catalyst for facile synthesis of 2,4,5-trisubstituted imidazole derivatives’, Monatsh. Chem. 2010, 141, 1339–1345.
- 30J. Safari, S. D. Khalili, S. H. Banitaba, ‘Three-component, one-pot synthesis of 2,4,5-trisubstituted imidazoles catalyzed by TiCl4−SiO2 under conventional heating conditions or microwave irradiation’, Synth. Commun. 2011, 41, 2359–2373.
- 31B. Maleki, H. Keshvari, A. Mohammadi, ‘Ammonium chloride: an effective catalyst for the one-pot synthesis of 2,4,5-trisubstituted imidazoles’, Orient. J. Chem. 2012, 28, 1207–1212.
- 32M. Ashrafi, A. Davoodnia, N. Tavakoli-Hoseini, ‘A Fast, Highly Efficient and Green Protocol for One-Pot Synthesis of 2,4,5-Trisubstituted Imidazoles Catalyzed by [TBA]2[W6O19] as a Reusable Heterogeneous Catalyst’, Bull. Korean Chem. Soc. 2013, 34, 1508–1512.
- 33H. V. Chavan, D. K. Narale, ‘Synthesis of 2,4,5-triaryl and 1,2,4,5-tetraaryl imidazoles using silica chloride as an efficient and recyclable catalyst under solvent-free conditions’, C. R. Chim. 2014, 17, 980–984.
- 34K. Nikoofar, M. Haghighi, M. Lashanizadegan, Z. Ahmadvand, ‘ZnO nanorods: Efficient and reusable catalysts for the synthesis of substituted imidazoles in water’, J. Taibah Univ. Sci. 2015, 9, 570–578.
- 35C. Yu, M. Lei, W. Su, Y. Xie, ‘Europium Triflate Catalyzed One-Pot Synthesis of 2,4,5-Trisubstituted-1H-imidazoles via a Three-component Condensation’, Synth. Commun. 2007, 37, 3301–3309.
- 36R. Mirsafaei, M. M. Heravi, S. Ahmadi, T. Hosseinnejad, ‘Synthesis and properties of novel reusable nano-ordered KIT-5-N-sulfamic acid as a heterogeneous catalyst for solvent-free synthesis of 2,4,5-triaryl-1H-imidazoles’, Chem. Pap. 2016, 70, 418–429.
- 37D. Wang, Z. Li, X. Huang, Y. Li, ‘Ce(SO4)2 ⋅ 4H2O as a highly efficient catalyst for the one-pot synthesis of tri- and tetrasubstituted imidazoles under solvent-free conditions’, ChemistrySelect 2016, 1, 664–668.
- 38T. Shamsi, A. Amoozadeh, E. Tabrizian, S. M. Sajjadi, ‘A new zwitterionic nano-titania supported Keggin phosphotungstic heteropolyacid: an efficient and recyclable heterogeneous nanocatalyst for the synthesis of 2,4,5-triaryl substituted imidazoles’, React. Kinet. Mech. Catal. 2017, 121, 505–522.
- 39V. Oliveira, M. Cardoso, L. Forezi, ‘Organocatalysis: A Brief Overview on Its Evolution and Applications’, Catalyst 2018, 8, 605.
10.3390/catal8120605 Google Scholar
- 40G. M. Ziarani, A. Badiei, N. Lashgari, Z. Farahani, ‘Efficient one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles using SBA−Pr−SO3H as a green nano catalyst’, J. Saudi Chem. Soc. 2016, 20, 419–427.
- 41A. A. Marzouk, V. M. Abbasov, A. H. Talybov, S. K. Mohamed, ‘Synthesis of 2,4,5-triphenyl imidazole derivatives using diethyl ammonium hydrogen phosphate as green, fast and reusable catalyst’, World J. Org. Chem. 2013, 1, 6–10.
- 42A. Mohammadi, H. Keshvari, R. Sandaroos, B. Maleki, H. Rouhi, H. Moradi, Z. Sepehr, S. Damavandi, ‘A highly efficient and reusable heterogeneous catalyst for the one-pot synthesis of tetrasubstituted imidazoles’, Appl. Catal. A 2012, 429, 73–78.
- 43N. Y. Yoon, C. Xie, K. B. Shim, Y. K. Kim, D. S. Lee, H. D. Yoon, ‘Antioxidant and Cholinesterase Inhibitory Activities of Antarctic Krill Eupausia superb’, Fish. Aquat. Sci. 2011, 14, 289–293.
10.5657/FAS.2011.0289 Google Scholar
- 44S. Aslam, S. Zaib, M. Ahmad, J. M. Gardiner, A. Ahmad, A. Hameed, N. Furtmann, M. Gütschow, J. Bajorath, J. Iqbal, ‘Novel structural hybrids of pyrazolobenzothiazines with benzimidazoles as cholinesterase inhibitors’, Eur. J. Med. Chem. 2014, 78, 106–117.
- 45C. Zhao, K. Rakesh, L. Ravidar, W. Y. Fang, H. L. Qin, ‘Pharmaceutical and medicinal significance of sulfur (SVI)-containing motifs for drug discovery: A critical review’, Eur. J. Med. Chem. 2018, 162, 679–734.
- 46B. V. Shitole, N. V. Shitole, S. B. Ade, G. K. Kakde, ‘Microwave-induced One-pot Synthesis of 2,4,5-trisubstituted Imidazoles Using Rochelle Salt as a Green Novel Catalyst’, Orbital: Electron. J. Chem. 2015, 7, 240–244.
- 47K. F. Shelke, S. Sapkal, S. Sonal, B. R. Madje, B. B. Shingate, M. S. Shingare, ‘An efficient synthesis of 2,4,5-triaryl-1H-imidazole derivatives catalyzed by boric acid in aqueous media under ultrasound-irradiation’, Bull. Korean Chem. Soc. 2009, 30, 1057–1060.
- 48M. S. Khan, S. A. Siddiqui, M. S. R. A. Siddiqui, U. Goswami, K. V. Srinivasan, M. I. Khan, ‘Antibacterial activity of synthesized 2,4,5-trisubstituted imidazole derivatives’, Chem. Biol. Drug Des. 2008, 72, 197–204.
- 49V. S. V. Satyanarayana, A. Sivakumar, ‘An efficient and novel one-pot synthesis of 2,4,5-triaryl-1H-imidazoles catalyzed by UO2(NO3)2 ⋅ 6H2O under heterogeneous conditions’, Chem. Pap. 2011, 65, 519–526.
- 50A. Hariharasubramanian, Y. D. Ravichandran, ‘Synthesis and studies of electrochemical properties of lophine derivatives’, RSC Adv. 2014, 54740–54746.
- 51G. L. Ellman, K. D. Courtney Jr., V. Andres, R. M. Featherstone, ‘A new and rapid colorimetric determination of acetylcholinesterase activity’, Biochem. Pharmacol. 1961, 7, 88–95.