Comparison of Chemical Profile and Antioxidant Capacity of Seeds and Oils from Salvia sclarea and Salvia officinalis
Jelena Živković
Institute for Medicinal Plant Research ‘Dr. Josif Pančić’, Tadeuša Košćuška 1, 11000 Belgrade, Serbia
Search for more papers by this authorMihailo Ristić
Institute for Medicinal Plant Research ‘Dr. Josif Pančić’, Tadeuša Košćuška 1, 11000 Belgrade, Serbia
Search for more papers by this authorJosephine Kschonsek
Institute of Nutrition, Friedrich Schiller University Jena, Dornburger Straße 25-29, 07743 Jena, Germany
Search for more papers by this authorAnna Westphal
Institute of Nutrition, Friedrich Schiller University Jena, Dornburger Straße 25-29, 07743 Jena, Germany
Search for more papers by this authorMilica Mihailović
Institute for Medicinal Plant Research ‘Dr. Josif Pančić’, Tadeuša Košćuška 1, 11000 Belgrade, Serbia
Search for more papers by this authorVladimir Filipović
Institute for Medicinal Plant Research ‘Dr. Josif Pančić’, Tadeuša Košćuška 1, 11000 Belgrade, Serbia
Search for more papers by this authorVolker Böhm
Institute of Nutrition, Friedrich Schiller University Jena, Dornburger Straße 25-29, 07743 Jena, Germany
Search for more papers by this authorJelena Živković
Institute for Medicinal Plant Research ‘Dr. Josif Pančić’, Tadeuša Košćuška 1, 11000 Belgrade, Serbia
Search for more papers by this authorMihailo Ristić
Institute for Medicinal Plant Research ‘Dr. Josif Pančić’, Tadeuša Košćuška 1, 11000 Belgrade, Serbia
Search for more papers by this authorJosephine Kschonsek
Institute of Nutrition, Friedrich Schiller University Jena, Dornburger Straße 25-29, 07743 Jena, Germany
Search for more papers by this authorAnna Westphal
Institute of Nutrition, Friedrich Schiller University Jena, Dornburger Straße 25-29, 07743 Jena, Germany
Search for more papers by this authorMilica Mihailović
Institute for Medicinal Plant Research ‘Dr. Josif Pančić’, Tadeuša Košćuška 1, 11000 Belgrade, Serbia
Search for more papers by this authorVladimir Filipović
Institute for Medicinal Plant Research ‘Dr. Josif Pančić’, Tadeuša Košćuška 1, 11000 Belgrade, Serbia
Search for more papers by this authorVolker Böhm
Institute of Nutrition, Friedrich Schiller University Jena, Dornburger Straße 25-29, 07743 Jena, Germany
Search for more papers by this authorAbstract
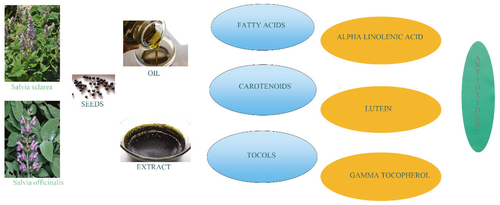
Composition of tocopherols, tocotrienols, carotenoids, fatty acids, as well as hydrophilic and lipophilic antioxidant activities, were determined in seeds of two Salvia species and oils obtained from them. Both seeds contained a large amount of oil (around 20%) rich in polyunsaturated fatty acids. While Salvia officinalis seed oil can be classified as oleic-linoleic oil, the predominant fatty acid in Salvia sclarea was α-linolenic acid (around 54%). Among tocols, the main isomers in both seeds and oils were γ-tocopherol, followed by α-tocopherol. Concerning carotenoids, their concentration was around 0.75 mg/100 g of seeds and 0.16 mg/100 g of oils, with a predominance of lutein. Oil and seeds of S. officinalis exhibited higher antioxidant potential compared to S. sclarea investigated samples which could be attributed to higher content of total vitamin E and carotenoids. This study provides results that enables use of two Salvia species as new alternative sources of vegetable oils.
Graphical Abstract
References
- 1M. Pourhosseini, J. Agarpanah, ‘Essential and Fixed Oil Chemical Profiles of Salvia aegyptiacca L. Flowers and Seeds’, J. Chil. Chem. Soc. 2015, 60, 2747 – 2748.
- 2S. Krimat, T. Dob, M. Toumi, L. Lamari, D. Dahmane, ‘Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oil of Salvia chundaei Batt. et Trab. Endemic Plant from Algeria’, J. Essent. Oil Res. 2015, 27, 447 – 453.
- 3N. U. Somer, B. B. Sarikaya, B. Erac, E. Kaynar, G. I. Kaya, M. A. Onur, B. Demirci, K. H. Can Baser, ‘Chemical Composition and Antimicrobial Activity of Essential Oils from the Aerial Parts of Salvia pinnata’, Rec. Nat. Prod. 2015, 9, 614 – 618.
- 4M. R. Loizzo, M. Abonali, P. Salehi, A. Sonboli, M. Kanani, F. Menichini, R. Tundis, ‘In vitro Antioxidant and Antiproliferative Activities of Nine Salvia Species’, Nat. Prod. Res. 2014, 28, 2278 – 2285.
- 5S. H. M. Farida, T. Radjabian, M. Ranjbar, S. A. Salami, N. Rahmani, A. Ghorbani, ‘Fatty Acid Patterns of Seeds of Some Salvia Species from Iran – A Chemotaxonomic Approach’, Chem. Biodiversity 2016, 13, 451 – 458.
- 6R. da Silva Marineli, E. A. Moraes, S. A. Lenquiste, A. T. Godoy, M. N. Eberlin, M. R. Maróstica Jr, ‘Chemical Characterization and Antioxidant Potential of Chilean Chia Seeds and Oil (Salvia hispanica L.)’, LWT – Food Sci. Technol. 2014, 59, 1304 – 1310.
- 7I. A. Nehdi, ‘Characteristics and Composition of Washingtoniaa fillifera (Linden ex André) H. Wendl. Seed and Seed Oil’, Food Chem. 2011, 126, 197 – 202.
- 8T. Costa, N. Jorge, ‘Characterization of Fatty Acids Profile of the Oils from Amazon Nuts and Walnuts’, Nutr. Food Sci. 2012, 42, 279 – 287.
10.1108/00346651211248647 Google Scholar
- 9V. Y. Ixtaina, M. L. Martínez, V. Spotorno, C. M. Mateo, D. M. Maestri, B. W. K. Diehl, S. M. Nolasco, M. C. Tomás, ‘Characterization of Chia Seed Oils Obtained by Pressing and Solvent Extraction’, J. Food Compos. Anal. 2011, 24, 166 – 174.
- 10M. Amato, M. C. Caruso, F. Guzzo, F. Galgano, M. Commissio, R. Bochicchio, R. Labella, F. Favati, ‘Nutritional Quality of Seeds and Leaf Metabolites of Chia (Salvia hispanica L.) from Southern Italy’, Eur. Food Res. Technol. 2015, 241, 615 – 625.
- 11C. L. Flakelar, P. D. Prenzler, D. J. Luckett, J. A. Howitt, G. Daran, ‘A Rapid Method for the Simultaneous Quantification of the Major Tocopherols, Carotenoids, Free and Esterified Sterols in Canola (Brassica napus) Oil Using Normal Phase Liquid Chromatography’, Food Chem. 2017, 214, 147 – 155.
- 12R. Ullah, M. Nadeem, A. Khalique, M. Imran, S. Mehmood, A. Javid, J. Hussain, ‘Nutritional and Therapeutic Perspectives of Chia (Salvia hispanica L.): A Review’, J. Food Sci. Technol. 2016, 53, 1750 – 1758.
- 13P. L. Zock, W. A. M. Blom, J. A. Nettleton, G. Hornstra, ‘Progressing Insights into the Role of Dietary Fats in the Prevention of Cardiovascular Disease’, Curr. Cardiol. Rep. 2016, 18, 111.
- 14S. Werner, V. Böhm, ‘Bioaccessibility of Carotenoids and Vitamin E from Pasta: Evaluation of an in Vitro Digestion Model’, J. Agric. Food Chem. 2011, 59, 1163 – 1170.
- 15M. J. Cook-Mills, C. A. McCary, ‘Isoforms of Vitamin E Differentialy Regulate Inflammation’, Endocr. Metab. Immune Disord. Drug Targets 2010, 10, 348 – 366.
- 16Y. Tang, X. Li, P. X. Chen, B. Zhang, M. Hernandez, H. Zhang, M. F. Marcone, R. Liu, R. Tsao, ‘Characterization of Fatty Acids, Carotenoids, Tocopherol/Tocotrienol Composition and Antioxidant Activities in Seeds of Three Chenopodium quinoa Willd. Genotypes’, Food Chem. 2015, 174, 502 – 508.
- 17H. Yalcin, I. Ozturk, E. Tulukcu, O. Sagdic, ‘Effects of γ-Irradiation on Bioactivity, Fatty Acids Composition and Volatile Compounds of Clary Sage Seed (Salvia sclarea L.)’, J. Food Sci. 2011, 76, C1056 – C1061.
- 18R. M. Bodoira, M. C. Penci, P. D. Ribotta, M. L. Martínez, ‘Chia (Salvia hispanica L.) Oil Stability: Study of the Effect of Natural Antioxidants’, LWT – Food Sci. Technol. 2017, 75, 107 – 113.
- 19C. F. Assumpção, I. L. Nunes, T. A. Mendonça, R. C. Bortolin, A. Jablanski, S. H. Flôres, A. de Oliveira Rios, ‘Bioactive Compounds and Stability of Organic and Conventional Grape Seed Oils’, J. Am. Oil Chem. Soc. 2016, 93, 115 – 124.
- 20G. Aruna, B. S. Mamatha, V. Baskaran, ‘Lutein Content of Selected Indian Vegetables and Vegetable Oils Determined by HPLC’, J. Food Compos. Anal. 2009, 22, 632 – 636.
- 21A. Ranalli, A. Sgaramella, G. Surricchio, ‘The New “Cytolase 0” Enzyme Processing Aid Improves Quality and Yields of Virgin Olive Oil’, Food Chem. 1999, 66, 443 – 454.
- 22R. A. Moreau, D. B. Johnston, K. B. Hicks, ‘A Comparison of the Levels of Lutein and Zeaxanthin in Corn Germ Oil, Corn Fiber Oil and Corn Kernel Oil’, J. Am. Oil Chem. Soc. 2007, 84, 1039 – 1044.
- 23Y. L. Kua, S. Gan, A. Morris, H. K. Ng, ‘A Validated, Rapid, Simple and Economical High-Performance Liquid Chromatography Method to Quantify Palm Tocopherol and Tocotrienols’, J. Food Compos. Anal. 2016, 53, 22 – 29.
- 24J. Karmowski, V. Hintze, J. Kschonsek, M. Killenberg, V. Böhm, ‘Antioxidant Activities of Tocopherols/Tocotrienols and Lipophilic Antioxdant Capacity of Wheat, Vegetable Oils, Milk Cream by Using Photochemiluminescence’, Food Chem. 2015, 175, 593 – 600.
- 25 Automated Mass Spectral Deconvolution and Identification System software (AMDIS ver. 2.64.), National Institute of Standards and Technology (NIST), Standard Reference Data Program, Gaithersburg, MD (USA).
- 26R. P. Adams, ‘ Identification of Essential Oil Components by Gas Chromatography /Mass Spectrometry’, 4th Edn., Allured Publishing Corporation, Carol Stream, Illinois, USA, 2007.
- 27J. Bauerfeind, V. Hintze, J. Kschonsek, M. Killenberg, V. Böhm, ‘Use of Photochemiluminescence for the Determination of Antioxidant Activities of Carotenoids and Antioxidant Capacities of Selected Tomato Products’, J. Agric. Food Chem. 2014, 62, 7452 – 7459.
- 28L. Müller, K. Fröhlich, V. Böhm, ‘Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (αTEAC), DPPH Assay and Peroxyl Radical Scavenging Assay’, Food Chem. 2011, 129, 139 – 148.
- 29J. Kschonsek, M. Stimming, L. Libuda, M. Kersting, V. Böhm, ‘Food-Based Modification of LC-PUFA Concentration in Complementary Food Did Not Affect Plasma Vitamin E Concentration in Infants’, NFS J. 2016, 3, 25 – 32.
10.1016/j.nfs.2016.02.005 Google Scholar
- 30T.-S. Shin, J. S. Godber, ‘Isolation of Four Tocopherols and Four Tocotrienols from a Variety of Natural Sources by Semi-Preparative High-Performance Liquid Chromatography’, J. Chromatogr. A 1994, 678, 49 – 58.
- 31L. Müller, S. Gnoyke, A. M. Popken, V. Böhm, ‘Antioxidant Capacity and Related Parameters of Different Fruit Formulations’, LWT – Food Sci. Technol. 2010, 43, 992 – 999.
- 32H.-C. Zhou, Y.-M. Lin, Y.-Y. Li, M. Li, S.-D. Wei, W.-M. Chai, N. F.-Y. Tam, ‘Antioxidant Properties of Polymeric Proanthocyanidins from Fruit Stones and Pericarps of Litchi chinensis Sonn’, Food Res. Int. 2011, 44, 613 – 620.