The Impact of Chemotherapy and Body Mass Index on Cancer-Related Fatigue in Colon Cancer Patients: A PROFILES-Registry Study
Funding: This research was supported by the Center of Research on Psychological Disorders and Somatic Diseases (CoRPS), Tilburg University, the Netherlands; the Netherlands Comprehensive Cancer Organisation, Utrecht, the Netherlands; and an Investment Subsidy Large (2016/04981/ZONMW-91101002) of the Netherlands Organisation for Scientific Research (The Hague, The Netherlands).
ABSTRACT
Background
Inflammation has been reported to drive cancer-related fatigue (CRF). As both obesity and chemotherapy promote inflammatory responses, obese cancer patients may be at risk of more severe CRF, especially when receiving chemotherapy.
Methods
We analysed data of 333 colon cancer patients from four hospitals in the Netherlands (data derived from the PROCORE study). Fatigue was assessed with the general fatigue subscale of the Multidimensional Fatigue Inventory at four timepoints: at inclusion (T1), 4 weeks after surgery (T2), and 1 (T3) and 2 years (T4) after diagnosis. Linear mixed-effects models were applied to evaluate the interaction between chemotherapy and body mass index (BMI) on the trajectory of fatigue.
Results
The two-way interactions between time and chemotherapy (p = 0.047) and between time and BMI on fatigue (p = 0.041) were significant. Patients scheduled for chemotherapy reported more fatigue during the treatment phase, while patients not treated with chemotherapy showed a stable trajectory. Obese patients reported more fatigue at follow-up compared to patients with a healthy BMI. The three-way interaction between time, chemotherapy and BMI was not significant (p = 0.39). However, obese chemotherapy-treated patients reported the highest fatigue 2 years after treatment (12.8, 95% CI: 10.6–14.9). Their mean fatigue score was higher compared to baseline (9.2, 95% CI: 7.3–11.0, p < 0.001) and obese patients not treated with chemotherapy (9.6, 95% CI: 7.0–12.2, p = 0.02). Moreover, this group reported more fatigue compared to healthy (8.1, 95% CI: 5.5–10.9, p < 0.001) and overweight (9.7, 95% CI: 7.2–12.3, p = 0.019) chemotherapy-treated patients.
Conclusion
This study indicates that chemotherapy and BMI both influence long-term fatigue in colon cancer patients. Proactive monitoring for CRF and lifestyle interventions may be needed for chemotherapy-treated patients with a high BMI.
Abbreviations
-
- AIC
-
- Akaike information criteria
-
- BMI
-
- body mass index
-
- CRF
-
- cancer-related fatigue
-
- MFI
-
- Multidimensional Fatigue Inventory
-
- NCR
-
- Netherlands Cancer Registry
-
- PROFILES
-
- Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship
1 Introduction
Colon cancer is one of the most common cancers in men and women worldwide, and the incidence is increasing [1]. As life expectancy of colon cancer patients increases steadily following improved early detection and treatment [2], quality of life after cancer treatment becomes more relevant for patients and clinicians.
One of the most common and burdensome complaints during and after cancer treatment is cancer-related fatigue (CRF) [3-5]. Patients with CRF experience a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion which is not proportional to recent activity, is hardly relieved by resting, and interferes with usual functioning [6]. As a result, cancer survivors suffering from CRF may be unable to regain their pre-cancer activity level and may face difficulties with daily life activities such as reading, getting out of bed, carrying out household chores, working or socialising [7]. CRF is most prominent during treatment but may persist for many years for a subset of colon cancer survivors [5].
While research efforts to understand CRF have increased in the past decades the pathophysiology of CRF remains unclear. A variety of potential biological mechanisms have been proposed, of which dysregulation of the immune system has been gaining much attention [8, 9]. Upregulation of pro-inflammatory cytokines as a result of chemotherapy treatment has been suggested to lead to CRF via central nervous system signalling [8, 10]. Longitudinal studies in colorectal cancer patients have shown that patients receiving chemotherapy score higher on CRF [11], and long-term CRF remains more common, compared to patients who did not receive chemotherapy [12]. Moreover, a recent study in breast cancer patients suggests that obese patients suffer more CRF compared to healthy-weight patients before, during and after chemotherapy [13]. Since obesity also can promote upregulation of pro-inflammatory cytokines and downregulation of anti-inflammatory cytokines [14], obese cancer patients may be at risk of more severe and prolonged CRF, especially when receiving chemotherapy.
To our knowledge, the interaction between chemotherapy and obesity has not yet been investigated in patients with colon cancer. Therefore, the aim of these secondary analyses on the PROCORE study was to evaluate the interaction between chemotherapy and Body Mass Index (BMI) on the level and trajectory of fatigue over time in colon cancer patients following their diagnosis. Exploring the interactions between chemotherapy and BMI may provide opportunities for focused (preventive/lifestyle-related) interventions to improve the quality of life of these cancer survivors.
2 Materials and Methods
2.1 Setting and Participants
This study is based on data derived from the PROCORE study. PROCORE was a prospective, population-based study aimed at examining the longitudinal impact of colon and rectal cancer and its treatment on patient-reported outcomes. Details of the data collection have been described previously [15]. In summary, the PROCORE study was performed by means of PROFILES, a registry for the physical and psychosocial impact of cancer and its treatment [16, 17]. PROFILES is linked to the Netherlands Cancer Registry (NCR), which collects clinical data from all newly diagnosed cancer patients in the Netherlands. Recruitment for PROCORE took place at four hospitals: Elisabeth-TweeSteden Hospital, Catharina Hospital, Elkerliek Hospital and Máxima Medical Centre. All patients newly diagnosed with primary colorectal cancer between January 2016 and January 2019 were approached and, if eligible and informed consent was provided, included shortly after diagnosis (i.e., before start of treatment). Patients previously diagnosed with cancer (except basal skin cell carcinoma), those with cognitive impairment and those unable to read or write Dutch, were excluded. In practice, a limited number of patients who were previously diagnosed with cancer and those who already started treatment were included. For this secondary analysis, patients with rectal cancer were excluded, since rectal cancer is clinically and biologically different from colon cancer [18]. Moreover, treatment differs between these types of cancer: radiotherapy is commonly administered to patients with rectal cancer, while patients with colon cancer are rarely treated with radiotherapy [19]. Since radiotherapy is known to have a (short-term) effect on CRF [20, 21] and the aim was to investigate the effect of chemotherapy, we excluded rectal cancer patients.
2.2 Data Collection
Eligible patients were invited by their research nurse or case manager and received an information package about the study. The package included an information letter, informed consent form and the first questionnaire (T1). Follow-up questionnaires (online or on paper) were sent 4 weeks after surgery (T2), and 1 (T3) and 2 years (T4) after diagnosis. In case of non-response, reminders were sent after 2 weeks. PROCORE was approved by the certified Medical Ethic Committee of Medical Research Ethics Committees United (registration number: NL51119.060.14).
2.3 Measures
In this analysis, participants were categorised based on their BMI at baseline: obese (≥ 30.0 kg/m2; n = 65), overweight (25.0–29.9 kg/m2; n = 159) and normal weight (18.0–24.9 kg/m2; n = 109). Participants with BMI < 18 kg/m2 were excluded from analysis. Height and weight (used to calculate BMI) were self-reported by patients. Patients' sociodemographic (i.e., age, sex) and clinical (i.e., cancer type, clinical stage, treatment) information was available from the NCR. Education level was derived from the questionnaire.
2.4 Fatigue
Fatigue was assessed with the General Fatigue scale of the Multidimensional Fatigue Inventory (MFI). This 20-item self-report instrument was designed to measure fatigue and has been validated in cancer patients [22]. The questionnaire has an equal amount of positively and negatively worded items which are rated on a 5-point Likert scale (e.g., “I feel tired”, “I feel rested”). It covers the five dimensions of fatigue: General Fatigue, Physical Fatigue, Mental Fatigue, Reduced Motivation and Reduced Activity. The General Fatigue subscale consists of 4 items, with the sum of scores ranging from 4 to 20. A higher score indicates a higher level of fatigue. For the MFI no absolute cut-off point for fatigue exists.
2.5 Statistical Analyses
Baseline characteristics were determined. Linear mixed effects models with maximum likelihood estimation and an unstructured covariance matrix with a 2-level structure (time-level and patient-level) were used. Mixed model analyses allow the number of observations per assessment to differ and therefore missing data were not imputed. A sequence of models was fitted to investigate fatigue from diagnosis until 2 years after diagnosis. Whether a more complex model fits the data better was determined based on the Akaike Information Criteria (AIC). A difference ≥ 4 between models was viewed as significant [23]. First, a model with no explanatory variables, only the intercept (i.e., an unconditional model), was fitted to the data to determine the extent of variance at the person and time level. Second, a model with only baseline characteristics (age, sex, education level) was calculated. Variables significantly associated with fatigue were used as covariates in the next models. Next, time as explanatory variable (unconditional growth model) was included to determine whether fatigue changed over time. Intercept and time were entered both as fixed and random effects as each subject may have its own unique intercept and slope. Third, the unconditional growth model was extended into a conditional growth model by including (i) possible covariates (i.e., age, sex, education level), (ii) chemotherapy (yes/no), (iii) baseline BMI and (iv) the interaction terms of time by chemotherapy, time by BMI and time by chemotherapy by BMI. By including the interaction terms, we were able to investigate whether fatigue develops differently over time depending on chemotherapy and BMI. Regression estimates and 95% confidence interval of fixed effects are presented. Analyses were done in SPSS version 23.
3 Results
In our analyses 333 eligible patients with colon cancer were included. Table 1 shows baseline clinical and demographic characteristics. Of all patients, 94 were treated with chemotherapy (28.2%). Relatively more males were included (60.0%). The majority of patients were overweight (47.7%) or obese (19.5%). Patients treated with and without chemotherapy were comparable with regard to age, BMI and education level. Between the different BMI categories patients were comparable with regards to age, education level and chemotherapy treatment. All patients completed baseline questionnaire, 292 (88%), 259 (78%) and 247 (74%) completed T2, T3 and T4, respectively.
| No. of patients (%) | |
|---|---|
| Total no. of patients | 333 |
| Age (SD) | 68.0 (9.14) |
| Sex | |
| Male | 200 (60.0%) |
| Female | 133 (39.9%) |
| Education level | |
| Low | 35 |
| Medium | 211 |
| High | 82 |
| Missing | 5 |
| Chemotherapy (yes) | 94 (28.2%) |
| Pathological tumour stage | |
| I | 108 (32.4%) |
| II | 108 (32.4%) |
| III | 109 (32.7%) |
| IV | 7 (2.1%) |
| X | 1 (0.3%) |
| BMI | |
| 18–25 | 109 (32.7%) |
| 25–30 | 159 (47.7%) |
| > 30 | 65 (19.5%) |
- Note: Education: low (no or primary school); medium (lower general secondary education or vocational training); high (pre-university education, high vocational training, university).
- Abbreviation: BMI, body mass index.
3.1 The Association Between Time and CRF
Intraclass correlation coefficients of the unconditional model showed that 56% of the variance was at the person level and the remaining 43% was at the time level. These results indicate that scores on CRF differed sufficiently between patients and over time to justify a two-level model. Next, we entered possible covariates (age, sex, education level) into the model. Only sex was found to be significantly different (p = 0.0035) indicating that women scored higher (10.1, 95% CI 9.4–10.8) on fatigue than men (9.0, 95% CI 8.4–9.7). Next, we computed an unconditional growth model by entering time as a fixed effect into the model. This model fitted the data better than the unconditional model and showed that time impacted the level of fatigue (p < 0.001). In this model, fatigue significantly increased from pre-treatment (9.2, 95% CI 8.6–9.6) to post-surgery (10.4, 95%CI 10.1–11.3) and decreased during follow-up at 1 (9.59, 95% CI 9.0–10.2) and 2 years (9.6, 95% CI 8.9–10.2; Figure 1, Table S1).
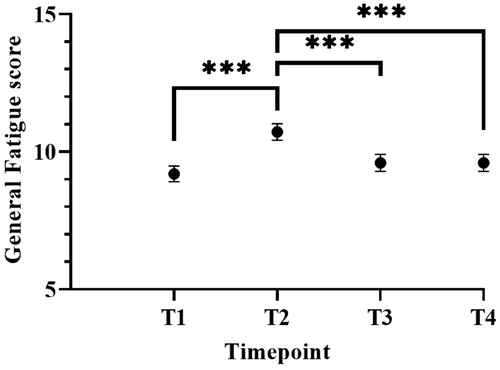
Moreover, the conditional growth model with time, sex, chemotherapy, BMI and the interaction terms showed that this model fitted the data as good as the unconditional growth model. A significant effect of time (p < 0.001), sex (p = 0.003), BMI (p = 0.009) and the interaction between time and chemotherapy (p = 0.047), and between time and BMI (p = 0.041) on fatigue levels was found. No significant three-way interaction between time, chemotherapy and BMI was found (p = 0.39).
3.2 The Interaction Between Time and Chemotherapy
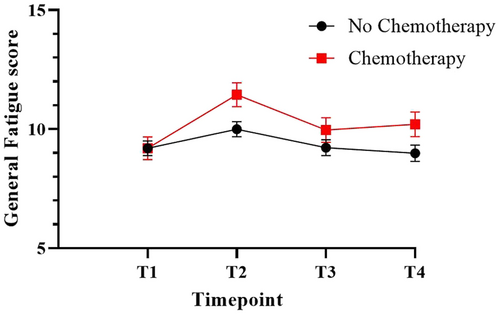
Chemotherapy-treated patients reported more fatigue during treatment phase (11.5, 95%CI 10.5–12.4) compared to pre-treatment (9.2, 95%CI 8.3–10.1), 1 year (10.0, 95%CI 9.0–11.0) and 2 year (10.2, 95%CI 9.2–11.2) follow-up (Figure 2 and Table S2). No significant differences over time are seen in the group that did not receive chemotherapy.
3.3 The Interaction Between BMI and Chemotherapy
The significant interaction between time and BMI shows that patients with a higher BMI reported a different fatigue trajectory compared to patients with a lower BMI (Figure 3, Table S3). Post hoc pairwise comparisons revealed that while no differences in fatigue are present between the BMI groups at baseline, differences arise during and after treatment. Overweight patients reported more fatigue during treatment phase compared to healthy weight patients (mean difference 1.6, p = 0.04). During follow-up, scores of overweight patients decrease again, while obese patients reported more fatigue 1 year (mean difference 2.2, p = 0.04) and 2 years after diagnosis (mean difference 2.6, p = 0.01) compared to patients with a healthy BMI.

3.4 Chemotherapy, BMI and the Trajectory of Fatigue
The three-way interaction between time, chemotherapy and BMI was not significant. However, post hoc analyses showed that the level of CRF at a specific assessment point does depend on BMI and chemotherapy. See Table 2 for an overview of the estimated effect of the covariates and interactions on the mean fatigue score. Chemotherapy-treated patients with a BMI ≥ 30 reported the highest levels of fatigue specifically at 2 years after diagnosis (Figure 4 and Table S4).
| Estimate | 95% CI | p | |
|---|---|---|---|
| Intercepta | 13.41 | 11.2, 15.6 | 0.000 |
| T1 | −3.59 | −5.6, −1.6 | 0.000 |
| T2 | −0.80 | −2.9, 1.3 | 0.456 |
| T3 | −1.16 | −3.3, 1 | 0.296 |
| Sex | −1.29 | −2.1, −0.5 | 0.003 |
| Chemotherapy | −3.20 | −5.8, −0.6 | 0.015 |
| BMI 18–25 | −4.62 | −7.3, −1.9 | 0.001 |
| BMI 25–30 | −3.06 | −5.6, −0.5 | 0.019 |
| T1 * Chemotherapy | 3.55 | 1.1, 6 | 0.004 |
| T2 * chemotherapy | 1.21 | −1.3, 3.7 | 0.344 |
| T3* chemotherapy | 1.17 | −1.4, 3.8 | 0.378 |
| T1 * BMI 18–25 | 3.94 | 1.4, 6.5 | 0.002 |
| T1 * BMI 25–30 | 3.81 | 1.4, 6.2 | 0.002 |
| T2 * BMI 18–25 | 2.91 | 0.3, 5.5 | 0.029 |
| T2 * BMI 25–30 | 3.22 | 0.7, 5.7 | 0.012 |
| T3 * BMI 18–25 | 1.42 | −1.3, 4.1 | 0.301 |
| T3 * BMI 25–30 | 1.33 | −1.2, 3.9 | 0.307 |
| T1 * Chemotherapy * BMI 18–25 | −0.12 | −3.1, 2.8 | 0.937 |
| T1 * Chemotherapy * BMI 25–30 | −0.93 | −3.7, 1.8 | 0.505 |
| T2 * Chemotherapy * BMI 18–25 | 1.03 | −2.1, 4.1 | 0.512 |
| T2 * Chemotherapy * BMI 25–30 | 0.56 | −2.4, 3.5 | 0.706 |
| T3 * Chemotherapy * BMI 18–25 | 2.24 | −1, 5.5 | 0.174 |
| T3 * Chemotherapy * BMI 25–30 | 1.63 | −1.4, 4.7 | 0.290 |
| T4 * Chemotherapy * BMI 18–25 | 3.15 | −0.1, 6.4 | 0.057 |
| T4 * Chemotherapy * BMI 25–30 | 2.80 | −0.3, 5.9 | 0.073 |
- Note: Post hoc results from the final conditional growth model by including sex, time, chemotherapy, baseline BMI, and the interaction terms of time by chemotherapy by BMI. The values in the table represent the estimated effect of the covariates and interactions on the mean fatigue score relative to the reference group: patients with a BMI ≥ 30 who received chemotherapy at T4. T1: at diagnosis, T2: 4 weeks after surgery, T3: 1 year post diagnosis, T4: 2 years after diagnosis.
- Abbreviation: BMI, body mass index.
- a BMI > 30 who received chemotherapy with mean fatigue score of 12.76 at T4.
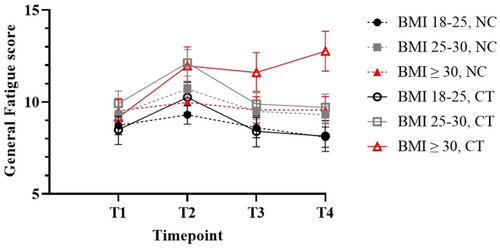
These patients reported a mean score of 12.8 (95%CI: 10.6–14.9) at T4, 3.6 point higher compared to baseline T1 (9.2, 95%CI: 7.3–11.0), p < 0.001. Patients with a BMI of 18–25 and 25–30 who received chemotherapy reported a significantly lower mean fatigue score of 8.1 (95%CI: 5.5–10.9, p < 0.001) and 9.7 (95%CI:7.2–12.3, p = 0.019), respectively, at T4. Moreover, chemotherapy-treated patients with a BMI ≥ 30 reported significantly higher levels of fatigue at T4 than their non-chemotherapy treated counterparts (9.6, 95%CI: 7.0–12.2, p = 0.015).
4 Discussion
CRF is a serious long-term effect of cancer treatment that dramatically decreases the quality of life of cancer survivors [6]. Previous research suggests that a high BMI and chemotherapy may interact to exacerbate CRF, but to what extent this applies to colon cancer patients remained unclear.
When analysing colon cancer patients as one group, fatigue increased during treatment phase, but recovered to pre-treatment levels during follow-up. Yet, when assessing the effects of chemotherapy and BMI, our study reveals several interesting observations. First, as expected, general fatigue was higher during treatment phase for chemotherapy-treated patients. As T2 was sent out 4 weeks after surgery, it is likely that not all patients scheduled for chemotherapy had started treatment when answering the questionnaire, potentially underrepresenting the effect of chemotherapy. Nonetheless, the interaction between time and chemotherapy showed that chemotherapy-treated patients were more fatigued during the treatment phase, while non-chemotherapy-treated patients showed a stable pattern. This is in line with existing literature in colorectal cancer patients [11, 12]. Second, overweight patients reported more fatigue during treatment phase compared to healthy weight patients. Obese patients remained more fatigued during follow-up compared to patients with a healthy BMI. This corroborates with earlier reported studies in colorectal cancer survivors that reported an association between higher BMI and fatigue [11, 24]. Thirdly, obese chemotherapy-treated patients did not recover to pre-treatment levels but rather continued to feel fatigued. Two years after treatment, this group of patients reported more fatigue compared to overweight (3.1 points higher) and healthy weight patients (4.6 points higher) as well as patients who did not receive chemotherapy at all (3.2 points higher). Given that a two-point difference on the MFI subscales is considered clinically meaningful, the observed long-term differences are substantial and of significant clinical relevance. These differences are highly pertinent for both patients and clinicians, as they may reflect considerable changes in functional status of cancer survivors [25]. The results are comparable with a recent study among 565 women with breast cancer treated with chemotherapy, reporting that obese patients (BMI ≥ 30) suffer more CRF compared to normal weight patients before, during and after chemotherapy [13]. Interestingly, BMI did not affect fatigue prior to treatment in our study. Differences may arise from differences in the time of measurement. In the breast cancer study, patients were assessed 7 days prior to chemotherapy and may have already been exposed to surgery and radiotherapy [13], while in our study patients had not received any treatment prior to the first measurement (T1). A high BMI itself does not appear to have a significant effect on MFI scores in healthy women [26]. The data in our study implies that the effect of BMI on fatigue is exacerbated when the patients are treated with chemotherapy, especially in the long term.
How the interplay of obesity and chemotherapy observed in this study results in an exacerbation of CRF in colon cancer patients remains to be clarified. Several lines of research have confirmed that obesity leads to chronic low-grade inflammation of the adipose tissue [27]. Weight gain and obesity cause a phenotypic switch of the adipose tissue, resulting in the release of proinflammatory cytokines [27]. Pro-inflammatory cytokines act on the central nervous system through several routes [28] and can elicit local inflammation resulting in altered brain functioning [29]. Conditions of chronic inflammation exacerbate sickness-like behaviours, characterised by poor sleep, reduced food intake, fatigue and pain complaints, social withdrawal, anhedonia, and memory and learning difficulties [28] that develop in response to acute peripheral inflammation, symptoms that are often seen with CRF. This phenomenon has been shown in several animal models with inflammatory conditions, and might be due to an effect called ‘priming’: exposure to a priming stimulus leads to an exaggerated production of pro-inflammatory cytokines when exposed to a triggering stimulus [28]. Preclinical experiments have shown that obese animals show a prolonged and more pronounced behavioural response when exposed to an inflammatory challenge [30, 31]. Chemotherapy might pose such an inflammatory challenge—alteration of cytokine profiles have been reported after standard doses of chemotherapy regimens [3, 32]. Disruption of the balance between pro– and anti-inflammatory cytokines following chemotherapy treatment may lead to a more intensified inflammatory response in obese cancer patients and result in chronic CRF. One may speculate that this may further be accelerated as obese patients may accumulate more drugs in fat tissues, resulting in a different release and altered inflammatory responses. Importantly, the effect of inflammation on brain functioning may be chronic, even if peripheral inflammation is not detectable anymore [33]. For this study, we analysed previously collected data from the PROCORE study. No data was currently available to identify biological pathways. Other mechanisms may play a role, such as alteration of gut microbiome, circadian rhythm disruption or HPA-axis dysregulation following chemotherapy [9]. Future (pre)clinical studies might examine the mechanisms of (neuro) inflammatory changes following chemotherapy treatment as well as the persistence of these changes by collecting biomarkers and proactive monitoring. Future research may also focus on lifestyle interventions to avoid fatigue exacerbation.
The value of this study includes the homogeneous sample of colon cancer patients as well as longitudinal design. The study has some limitations. No data was available on the chemotherapy type or dosing schedule. Higher body weight likely resulted in higher absolute dosing, as patients are dosed according to body surface area. Fatigue may therefore result from exposure to a higher dose of chemotherapy which we have not been able to account for. Moreover, weight and height were self-reported and may be subject to response bias. In addition, it is not known why patients were lost to follow-up, as such the effect of it on our analysis remains unclear. Furthermore, a very small number of patients received chemotherapy prior to the baseline questionnaire, which could have impacted the strength of our findings. Finally, the present results may not be generalisable to a broader group of (gastrointestinal) cancer patients. Due to the relatively low number of stage IV patients who participated in the study, our findings should be extrapolated to this group with care. Nevertheless, this study shows that further investigation of the interaction between chemotherapy, BMI and CRF is indispensable.
5 Conclusion
Our study indicates that colon cancer patients with a BMI ≥ 30 who receive chemotherapy treatment have a higher risk of remaining fatigued over longer periods, in contrast to patients that do not receive chemotherapy treatment or are not obese. Regular monitoring for CRF in this group as well as proactive communication with these patients on the possibility that their BMI may impact chronic fatigue may be needed. Moreover, lifestyle modifying coaching, including dietary and exercise interventions, may be beneficial prior, during or after treatment.
6 Précis
Results from this study indicate that colon cancer patients with a BMI≥ 30 develop chronic fatigue after treatment with chemotherapy. Two years after treatment, these patients reported significantly higher fatigue levels compared to patients with a lower BMI or patients who did not receive chemotherapy at all.
Author Contributions
Anneke Kastelein: conceptualisation, methodology, writing – original draft, visualisation. Floortje Mols: methodology, investigation, resources, writing – review and editing. Laura Kervezee: validation, writing – review and editing. Niels H. Chavannes: supervision, writing – review and editing. Hans Gelderblom: supervision, writing – review and editing. Jacques Neefjes: supervision, writing – review and editing. Chris Hinnen: conceptualisation, methodology, formal analysis, writing – original draft.
Acknowledgements
We would like to thank all patients and their physicians for their participation in PROCORE. Special thanks go to C. Rolf, MD, and F. van Heest, MD, who were willing to function as independent advisors and to answer questions of patients. In addition, we want to thank the following hospitals for their collaboration: Elisabeth-TweeSteden Hospital, Tilburg; Catharina Hospital, Eindhoven; Elkerliek Hospital, Helmond; Máxima Medical Centre, Eindhoven and Veldhoven.
Ethics Statement
The PROCORE study was approved by the certified Medical Ethic Committee of Medical Research Ethics Committees United (registration number: NL51119.060.14).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data is freely available for non-commercial scientific research, subject to study question, privacy and confidentiality restrictions, and registration (www.profilesregistry.nl).