Prognostic factors in patients with localized and metastatic alveolar rhabdomyosarcoma. A report from two studies and two registries of the Cooperative Weichteilsarkom Studiengruppe CWS
Ewa Koscielniak and Sabine Stegmaier should be considered joint first author.
Abstract
Background
The histologic classification of rhabdomyosarcoma (RMS) as alveolar (aRMS) or embryonal (eRMS) is of prognostic importance, with the aRMS being associated with a worse outcome. Specific gene fusions (PAX3/7::FOXO1) found in the majority of aRMS have been recognized as markers associated with poor prognosis and are included in current risk stratification instead of histologic subtypes in localized disease. In metastatic disease, the independent prognostic significance of fusion status has not been definitively established. The objective of this analysis was to evaluate survival outcomes of patients with localized and metastatic aRMS and its association with fusion status and subtype (PAX3/7::FOXO1, FOXO1 break), and clinical prognostic factors.
Methods
A total of 470 patients with aRMS ≤21 years of age enrolled in two CWS-trials and two registries was eligible for the analysis.
Results
The 5-year event-free survival (EFS) and overall survival (OS) rates for all patients with localized vs. metastatic tumors were: 56% and 65% vs. 18% and 22%, respectively. Of the 368 (78%) tumors tested, specific fusion was found in 330 (90%), considered “fusion positive” FP (PAX3::FOXO1 in 280, PAX7::FOXO1 in 49, FOXO1 break in 59 tumors). In patients with localized tumors, univariate analysis revealed that clinical group, tumor invasiveness (T1 vs.T2), regional lymph node involvement (N0 vs. N1) and FOXO1 fusion were significantly associated with EFS and OS, tumor size and PAX variant with OS only. In patients with metastatic aRMS, age, bone/marrow (B/BM) metastases, FOXO1 fusion and PAX variant were associated with EFS and OS, T status with OS only. Multivariate analysis identified PAX3::FOXO1 fusion as an independent adverse prognostic factor for EFS in patients with localized disease and for EFS and OS in patients with metastatic disease, B/BM metastases for EFS.
Conclusion
PAX3::FOXO1 fusion should replace FOXO1 fusion as an adverse prognostic factor in risk stratification. The prognostic relevance of PAX7::FOXO1-positive and FOXO1 fusion negative aRMS, along with the clinical factors described in this report, allows further refinement of risk assessment of patients with localized and metastatic aRMS.
1 INTRODUCTION
Rhabdomyosarcomas (RMS) are a heterogeneous group of malignancies of mesenchymal cell origin and are the most common soft tissue sarcoma (STS) of childhood accounting for more than 50% of all cases in patients aged <18 (kinderkrebsregister.de).
The cure rate of children with localized RMS has increased from 25% in 1970 to over 70% in 1990.1, 2 In the last three decades, however no further significant improvements in survival rates have been achieved but the cumulative therapy burden has become better adapted to risk profile allowing for minimization of severe late effects.3, 4 An exception is the RMS 2005 study of the European pediatric Soft Tissue Sarcoma Study Group (EpSSG), which showed a 13% improvement in OS with maintenance therapy in patients with high-risk RMS.5 In patients with metastatic disease outcome has not improved significantly and remains poor.6, 7 Factors defined as directly influencing prognosis, such as patient age, histologic subtype, tumor site, size, invasiveness, resectability and regional lymph node involvement have been incorporated into risk-adapted stratification systems for patients with localized RMS.4, 8, 9 In metastatic disease, factors associated with prognosis have been defined but have not been widely used to stratify therapy.10, 11 In the pediatric population two major RMS subtypes exist: “alveolar” (aRMS) and “embryonal” (eRMS), whereas tumors with pleomorphic and spindle cell/sclerosing cell histology account for the majority of RMS diagnosed in individuals >18 years of age. Chromosomal translocations resulting in fusion of the DNA-binding domain of the PAX3 or PAX7 genes to the transactivation domain of the FOXO1 gene have been detected in approximately 80% of aRMS and a small subset express rare variants such as PAX3::FOXO4 or PAX3::NCOA1/2.12-15 aRMS expressing either PAX::FOXO1 gene share a common expression profile distinct from fusion-negative aRMS and from other RMS variants.16, 17 aRMS subtype was known as being strongly associated with poor prognosis and was regarded in the past as a “unfavorable” histology in risk stratification for patients with localized tumors.3, 4, 18 However, histologic subtype was not an independent prognostic factor in an analysis of patients with metastatic disease treated in nine trials conducted by European and American cooperative groups.11 Several studies over the past 20 years have reported that the presence of the FOXO1-fusion, rather than alveolar histology, is associated with poor outcome.17, 19-21 FOXO1 status has been shown to be the most important prognostic factor in patients with RMS after metastatic status in an analysis of six Children's Oncology Group (COG) clinical trials and is included in risk stratification for patients with localized disease of the ongoing COG and EpSSG trials (COG ARST1431 NCT02567435 and EpSSG FaR-RMS NCT 04625907).22 In metastatic disease, the independent prognostic significance of fusion status has not been definitively established.6, 23 However, most analyses evaluating clinical factors influencing outcome have included patients with all histologically or molecularly defined subtypes of RMS.9, 22
In the recently published European studies, all patients with localized aRMS were included in the high and very high (patients with lymph node involvement) risk groups, regardless of other known clinical risk factors.3, 4 Similarly, patients with FOXO1 fusion-positive localized RMS without lymph node involvement will not be further stratified in the ongoing EpSSG and COG trials.9 More recently, an analysis of patients with localized FOXO1 fusion-positive RMS treated in three COG trials has shown that this group can be further stratified based on clinical characteristics.24 However, it has not yet been investigated which clinical factors, in addition to fusion status and subtype, influence the prognosis of patients with aRMS. The purpose of this analysis was therefore to evaluate survival outcomes of patients with localized and metastatic aRMS and its association with fusion status (PAX3/7::FOXO1, FOXO1 break) and type (PAX3- vs. PAX7::FOXO1) and clinical prognostic factors in patients enrolled in two studies: CWS-2002P, CWS-IV 2002, and two registries: CWS-DOK IV 2004 and the European Soft Tissue Sarcoma Registry SoTiSaR.
2 MATERIALS AND METHODS
Patients with aRMS ≤21 years of age with sufficient clinical data registered in two trials (CWS-2002P 17.1.2003-31.12.2010, CWS-IV 2002 10.1.2005-31.12.2007) and two registries (CWS-DOK IV 10.1.2005-31.12.2010, SoTiSaR 1.7.2009-31.12.2017) were eligible for the analysis. Patients with localized eRMS from the same trials were included in the survival analysis only as a reference group (Figure 1). CWS-2002P was designed as prospective, non-randomized, historically controlled trial on localized RMS and non-rhabdomyosarcoma soft tissue sarcoma (NRSTS).4 CWS-IV 2002 was an open Phase II window study and CWS-DOK IV 2004 was a prospective registry for patients aged ≤21 years with metastatic soft tissue sarcoma (STS).25, 26 SoTiSaR is a prospective European Registry for STS in patients aged ≤21 years. Participating countries are Austria, Germany, Poland, Sweden, Switzerland and Finland. Therapy details of CWS-2002P and CWS-IV 2002, CWS-DOK-IV 2004 and CWS-Guidance (treatment recommendations for patients enrolled on SoTiSaR) have already been published.4, 6, 25-27 Briefly: patients with localized aRMS were assigned to the high and very high-risk group. Risk stratification is shown in Table S1. Patients with metastatic disease were not further stratified. All patients received multimodal treatment with chemotherapy, surgery and/or radiotherapy according to the respective protocol, patients enrolled in the SoTiSaR according to the CWS-Guidance. Chemotherapy consisted of three or four drugs (ifosfamide, vincristine, actinomycin and doxorubicin). In patients with metastatic tumors, doxorubicin was replaced by eprirubicin and etoposide and carboplatin were added. More information on the details of therapy is available in the Supporting information (Methods, Figures S1 and S2). Informed consent was obtained from all parents/guardians or patients according to the legal requirements and the Declaration of Helsinki. CWS-2002P, CWS-IV 2002 and SoTiSaR were approved by Ethics Review Board of the University of Tübingen (51/2003, 218/2000 and 158/2009B02). Ethical review and approval were waived for CWS-DOK IV 2004, as it was considered an addendum to the CWS-2002P. All diagnoses were confirmed by central pathology review (C.V).In addition to the typical morphology of aRMS, P-cadherin positivity, with the absence of EGFR staining and documentation of a sparse reticulin fiber network by reticulin staining, was required to make a diagnosis of aRMS.12 Molecular testing was used to detect PAX3/7::FOXO1 or PAX3::NCOA1/2 by RT-PCR and/or FISH (FOXO1 break) and was performed centrally (S.S. and C.V) as previously described.12, 28-31 aRMS negative in both tests were described as “fusion negative” FN aRMS. If the testing was not performed the tumor was diagnosed based on morphological criteria only. C.V conducted a histology re-review of all cases classified as alveolar but lacking fusion transcripts. In 8 FN aRMS cases, tumor samples were available for RNA sarcoma panel sequencing, which did not reveal any other rare fusions. Rare fusions known to occur in aRMS were included in the panel. Details on genes of the panel are available in the Data S1.
2.1 Statistical methods
Statistical analyses were performed using SPSS statistics 22–25.0.0 (IBM Corporation. Armonk. NY) and R 3.02 (Bell Laboratories. Murray Hill. NJ. USA) software packages. Continuous variables were summarized by indicating their interquartile ranges whereas categorical variables were reported as counts and percentages. Clinical variables and its frequency distributions were compared by the χ2-test or by the Fisher-test. Event free survival (EFS) and overall survival (OS) were calculated using Kaplan–Meier method. EFS was calculated as the time elapsed between the date of diagnosis and either the occurrence of an event or the date of the last patient contact. Event was defined as relapse of disease (local, metastatic or combined) in patients who achieved complete remission, disease progression (defined as growth of tumor in patients who did not achieved complete remission) or death. Second malignancy was not defined as an event as described in the protocols. OS was defined as time from diagnosis to death or last follow up for surviving patients. Patients who had not experienced an event at their last contact were considered censored. Difference in outcome rates (survival curves) were estimated by the log-rank test statistic (Mantel–Haenszel Test) at an alpha level of 0.05 (5%). Multivariate analysis was performed using Cox's proportional hazards to simultaneously assess the effect of the variables tested in the univariate analysis, with the exception of tumor site, the inclusion of which resulted in numerical instability “loklik converged before variabe 3,4 coefficient may be infinite”. Fusion-positive tumors could not be analyzed as a whole group in the multivariate analysis when evaluated in strata due to their correlation (to avoid multicollinearity). The proportional hazards assumption was tested by checking the Schoenfeld residuals.
3 RESULTS
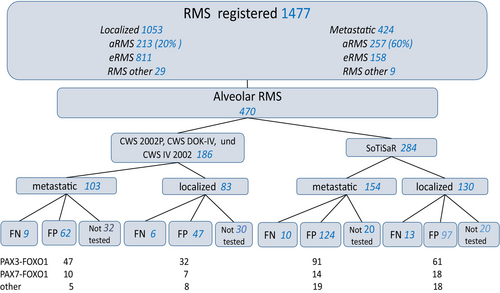
Eligibility criteria were fulfilled in 470 patients (213 with localized and 257 with metastatic disease). The slightly higher number of patients with metastatic versus localized disease reflects the known much higher incidence of the alveolar subtype in metastatic RMS.
The distribution of patients by study and disease extent is shown in Figure 1. Patient characteristics are presented in Table 1.
| Variable | Localized | Metastatic | Total | |||
|---|---|---|---|---|---|---|
| 213 | % | 257 | % | 470 | % | |
| Gender | ||||||
| Male | 104 | 48.8 | 126 | 49.0 | 230 | 48.9 |
| Female | 108 | 50.7 | 130 | 50.6 | 238 | 50.6 |
| Missing data | 1 | 0.5 | 1 | 0.4 | 2 | 0.4 |
| Age | ||||||
| ≤10 years | 126 | 59.2 | 98 | 38.1 | 224 | 47.7 |
| >10 years | 87 | 40.8 | 159 | 61.9 | 246 | 52.3 |
| Tumor size | ||||||
| ≤5 cm | 106 | 49.8 | 61 | 23.7 | 167 | 35.5 |
| >5 cm | 91 | 42.7 | 165 | 64.2 | 256 | 54.5 |
| Missing data | 16 | 7.5 | 31 | 12.1 | 47 | 10.0 |
| Tumor site | ||||||
| EXT | 68 | 31.9 | 119 | 46.3 | 187 | 39.8 |
| HN-nPM | 39 | 18.3 | 3 | 1.2 | 42 | 8.9 |
| HN-PM | 55 | 25.8 | 31 | 12.1 | 86 | 18.3 |
| ORBITA | 8 | 3.8 | 0 | 0.0 | 8 | 1.7 |
| GU-BP | 5 | 2.3 | 13 | 5.1 | 18 | 3.8 |
| GU-nBP | 7 | 3.3 | 5 | 1.9 | 12 | 2.6 |
| Other | 31 | 14.6 | 79 | 30.7 | 110 | 23.4 |
| Missing data | 0 | 0.0 | 7 | 2.7 | 7 | 1.5 |
| Tumor Site Risk group | ||||||
| Favorable (ORB, GU-nBP, HN-nPM)a | 54 | 25.3 | 8 | 3.1 | 62 | 13.2 |
| Unfavorable (EXT, HN-PM, GU-BP, Other)b | 159 | 74.7 | 242 | 94.2 | 401 | 85.3 |
| Missing data | 0 | 0 | 7 | 2.7 | 7 | 1.5 |
| IRS Group | ||||||
| I | 10 | 4.7 | 0 | 0.0 | 10 | 2.1 |
| II | 29 | 13.6 | 0 | 0.0 | 29 | 6.2 |
| III | 174 | 81.7 | 0 | 0.0 | 174 | 37.0 |
| IV | 0 | 0.0 | 257 | 100.0 | 257 | 54.7 |
| Risk group | ||||||
| High | 136 | 63.8 | 0 | 0.0 | 136 | 28.9 |
| Very high | 75 | 35.2 | 0 | 0.0 | 75 | 16.0 |
| Metastatic | 0 | 0.0 | 257 | 100.0 | 257 | 54.7 |
| Missing data | 2 | 0.9 | 0 | 0.0 | 2 | 0.4 |
| T-status | ||||||
| T1 | 99 | 46.5 | 34 | 13.2 | 133 | 28.3 |
| T2 | 95 | 44.6 | 205 | 79.8 | 300 | 63.8 |
| Tx (missing data) | 19 | 8.9 | 18 | 7.0 | 37 | 7.9 |
| N-status | ||||||
| N0 | 126 | 59.2 | 53 | 20.6 | 179 | 38.1 |
| N1 | 75 | 35.2 | 160 | 62.3 | 235 | 50.0 |
| NX (missing data) | 12 | 5.6 | 44 | 17.1 | 56 | 12.0 |
| B/BM | ||||||
| Yes | 158 | 61.5 | ||||
| No | 93 | 36.2 | ||||
| Missing data | 6 | 2.3 | ||||
| Study | ||||||
| SoTisaR | 130 | 61.0 | 154 | 59.9 | 284 | 60.4 |
|
CWS-2002P, CWS-DOK IV CWS-IV 2004 |
83 | 39.0 | 103 | 40.1 | 186 | 39.6 |
| FOXO1 fusion status | ||||||
| Negative | 19 | 8.9 | 19 | 7.4 | 38 | 8.1 |
| Positive | 144 | 67.6 | 186 | 72.4 | 330 | 70.2 |
| Not known | 50 | 23.5 | 52 | 20.2 | 102 | 21.7 |
- a ORB, orbita; GU-nBP, genitourinary-non bladder/prostate; HN-nPM, head/neck-non parameningeal.
- b EXT, extremity; HN-PM, head/neck-parameningeal; GU-BP, genitourinary-bladder/prostate.
Median follow-up for all patients was 8.3 years (IQR 5.3–10.7). The estimated 5-year EFS and OS rates for all patients with localized tumors were: 56% (95% CI 49–63) and 65% (95% CI 58–72), for patients with metastatic disease 18% (95% CI 14–24) and 22% (95% CI 17–28) respectively. There was no difference in EFS and OS between the different CWS cohorts (Tables 2 and 3). 368 tumors (78%) were tested by FISH and/or RT-PCR, of which the specific fusion was found in 330 (90%) aRMS (“fusion positive” FP). 38 tumors (10%) were classified as “fusion negative” FN aRMS. In 102 (22%) tumors the fusion status was not known. The PAX variant was available for 280 (85%) FP tumors, with PAX3::FOXO1 representing the vast majority (n = 231, 82%). PAX7::FOXO1 was found in 49 tumors (18%). In 50 the FOXO1 fusion partner was not known. No PAX3::NCOA1/2 fusion was found in the cohort studied. There was no difference in the distribution of patient and tumor-related variables between patients with FP and FN localized aRMS, in contrast to patients with metastatic tumors, where age >10 years, unfavorable primary sites and bone and/or bone marrow (B/BM) involvement were significantly more frequent in the FP group (Tables S2 and S3).
| Variable | n | % | 5 year EFS % (95% CI) | p logrank | 5 year OS % (95% CI) | p logrank |
|---|---|---|---|---|---|---|
| Study | ||||||
| SoTiSaR | 130 | 61.0 | 54.6 (45.9–65.0) | 0.39 | 65.2 (56.1–75.7) | 0.95 |
| CWS-2002P | 83 | 39.0 | 58.7 (48.8–70.6) | 63.9 (54.0–75.6) | ||
| Age | ||||||
| ≤10 | 126 | 59.2 | 57.7 (49.0–67.9) | 0.37 | 65.1 (56.3–75.4) | 0.46 |
| >10 | 87 | 40.8 | 53.6 (43.6–65.9) | 64.1 (54.0–76.0) | ||
| Tumor size | ||||||
| ≤5 cm | 106 | 49.8 | 60.9 (51.7–71.8) | 0.24 | 70.9 (61.7–81.4) | 0.04 |
| >5 cm | 91 | 42.7 | 52.1 (42.3–64.2) | 60.4 (50.3–72.4) | ||
| Missing data | 16 | 7.5 | ||||
| Tumor site | ||||||
| Favorablea | 54 | 25.4 | 57.2 (44.7–73.2) | 0.95 | 70.1 (57.6–85.5) | 0.23 |
| Unfavorableb | 159 | 74.6 | 55.7 (48.0–64.6) | 62.8 (54.9–71.8) | ||
| IRS-group | ||||||
| I | 10 | 4.7 | 76.2 (52.1–100.0) | 0.05 | 80.0 (51.6–100.0) | 0.05 |
| II | 19 | 8.9 | 76.3 (61.4–94.9) | 83.1 (69.2–99.8) | ||
| III | 174 | 81.7 | 51.8 (44.4–60.4) | 60.7 (53.1–69.4) | ||
| T-status | ||||||
| T1 | 99 | 46.5 | 66.0 (56.7–76.9) | 0.07 | 75.3 (66.1–85.7) | 0.003 |
| T2 | 95 | 44.6 | 46.6 (37.2–58.4) | 54.1 (44.2–66.3) | ||
| TX | 19 | 8.9 | ||||
| N-status | ||||||
| N0 | 126 | 59.2 | 50.2 (42.9–58.8) | 0.002 | 56.4 (48.8–65.2) | 7.0e-04 |
| N1 | 75 | 35.2 | 28.1 (22.6–35.0) | 33.1 (27.1–40.5) | ||
| NX | 12 | 5.6 | ||||
| Fusion status | ||||||
| Positive | 144 | 67.6 | 47.8 (39.9–57.4) | 0.002c | 59.5 (51.0–69.4) | 0.009c |
| Negative | 19 | 8.9 | 93.7 (82.6–100.0) | 93.7 (82.6–100.0) | ||
| Not known | 50 | 23.5 | −63.3 (49.7–80.5) | 71.4 (8.1–87.8) | ||
| Pax variant | ||||||
| PAX3 :: FOXO1 | 93 | 43.7 | 42.0 (32.8–53.8) | 0.15 | 50.5 (40.4–63.1) | 0.03d |
| PAX7 :: FOXO1 | 25 | 11.7 | 60.4 (43.1–84.7) | 80.9 (65.6–99.7) | ||
| PAX not known | 26 | 12.2 | 66.0 (48.1–90.5) | 68.4 (50.1–93.3) | ||
- a ORB, orbita; GU-nBP, genitourinary-non bladder/prostate; HN-nPM, head/neck-non parameningeal.
- b EXT, extremity; HN-PM, head/neck-parameningeal; GU-BP, genitourinary-bladder/prostate.
- c p for positive versus negative.
- d p for PAX3 versus PAX7.
| n (257) | % | 5 year EFS % (95% CI) | p logrank | 5 year OS % (95% CI) | p logrank | |
|---|---|---|---|---|---|---|
| Study | ||||||
| SoTiSaR | 154 | 59.9 | 18.1 (12.6–26.2) | 0.79 | 21.7 (15.4–30.6) | 0.83 |
| CWS-IV-2002 CWS-DOK IV | 103 | 40.1 | 17.9 (11.7–27.4) | 21.3 (14.6–31.3) | ||
| Age | ||||||
| ≤10 | 98 | 38.1 | 32.1 (23.5–43.9) | 6.3 e-04 | 39.0 (29.7–51.3) | 6.8 e-05 |
| >10 | 159 | 61.9 | 9.9 (6.1–16.2) | 11.6 (7.2–18.6) | ||
| Gender | ||||||
| Male | 130 | 50.6 | 21.2 (14.9–30.2) | 0.30 | 22.0 (15.4–31.5) | 0.99 |
| Female | 126 | 49.0 | 15.4 (10.0–23.7) | 21.4 (14.9–30.7) | ||
| Missing data | 1 | 0.4 | ||||
| Tumor size | ||||||
| ≤5 cm | 61 | 23.7 | 18.1 (10.4–31.6) | 0.41 | 21.8 (13.0–21.6) | 0.45 |
| >5 cm | 165 | 64.2 | 20.3 (14.7–27.9) | 24.3 (18.2–32.5) | ||
| Missing data | 31 | 12.0 | ||||
| Tumor site | ||||||
| Favorablea | 8 | 3.1 | 29.2 (9.1–93.2) | 0.30 | 33.3 (10.8–100.0) | 0.20 |
| Unfavorableb | 242 | 94.2 | 17.9 (13.4–23.8) | 21.7 (16.7–28.1) | ||
| Missing data | 7 | 2.7 | ||||
| T-status | ||||||
| T1 | 34 | 13.2 | 25.3 (13.9–46.0) | 0.20 | 44.1 (29.4–66.2) | 0.05 |
| T2 | 205 | 79.8 | 18.1 (13.2–24.7) | 19.8 (14.6–26.7) | ||
| Missing data | 18 | 7.0 | ||||
| N-status | ||||||
| N0 | 53 | 20.6 | 11.4 (4.9–26.5) | 0.40 | 15.0 (7.3–30.7) | 0.40 |
| N1 | 160 | 62.3 | 20.6 (14.9–28.4) | 24.1 (17.8–32.5) | ||
| Missing data | 44 | 17.0 | ||||
| B/BMc metastases | ||||||
| Yes | 159 | 61.9 | 11.4 (7.2–18.1) | 6.0e-05 | 12.2 (7.8–19.1) | 4.0e-05 |
| No | 91 | 35.4 | 30.3 (21.6–42.4) | 37.6 (28.0–50.0) | ||
| Missing data | 8 | 2.7 | ||||
| Fusion status | ||||||
| Positive | 186 | 72.4 | 10.8 (7.0–16.7) | 6.0 e-06d | 14.8 (10.2–21.5) | 7.0e-05d |
| Negative | 19 | 7.4 | 71.0 (52.3–96.4) | 70.1 (51.0–96.1) | ||
| Not known | 52 | 20.2 | 27.5 (17.0–44.5) | 30.1 (18.8–48.5) | ||
| Fusion variant | ||||||
| PAX 3 :: FOXO1 | 138 | 53.7 | 7.7 (4.2–14.0) | 0.04 | 9.8 (5.6–17.1) | 0.03e |
| PAX 7 :: FOXO1 | 24 | 9.3 | 17.2 (6.6–44.7) | 28.3 (14.1–56.9) | ||
| PAX not known | 24 | 9.3 | 25.2 (12.1–52.4) | 29.3 (15.0–57.2) | ||
- a ORB, orbita; GU-nBP, genitourinary-non bladder/prostate; HN-nPM, head/neck-non parameningeal.
- b EXT, extremity; HN-PM, head/neck-parameningeal; GU-BP, genitourinary-bladder/prostate.
- c B/BM, bone/bone marrow.
- d p for positive versus negative.
- e p for PAX3 versus PAX7.
When comparing clinical characteristics between PAX3:: vs. PAX7::FOXO1 positive localized aRMS, the only difference was a higher percentage of non-invasive tumors (“T1”) in PAX7::FOXO1 group (Table S4). In metastatic disease, patients with PAX7::FOXO1 aRMS were significantly younger compared to PAX3::FOXO1 positive group (68% vs. 33% of patients ≤10 years of age), all other factors were similarly distributed (Table S5).
On univariate analysis in patients with localized disease, IRS (Intergroup Rhabdomyosarcoma Study) group, regional lymph node involvement (N0 vs.N1) and FOXO1 fusion status were associated with EFS and OS (Table 2). Patients with IRS group III, N1, and FP tumors had significantly inferior EFS and OS compared to those with IRS group I-II, N0 status and FN aRMS. For tumor size, tumor invasiveness (T1 vs. T2) and FOXO1 fusion partner (PAX3 vs. PAX7) a significant correlation was observed for OS (Table 2). Patients with invasive (T2), large (>5 cm) tumors and bearing PAX3::FOXO1 fusion had significantly inferior OS compared to those with non-invasive (T1), smaller ≤5 cm and PAX7::FOXO1 positive tumors. In multivariate analysis, only PAX3::FOXO1 fusion status emerged as independent prognostic factor for EFS (Table 4).
| Localizeda | Metastaticb | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | HR EFS | 95% CI | p-value | HR OS | 95% CI | p-value | n | HR EFS | 95% CI | p-value | HR OS | 95% CI | p-value | |
| Age | ||||||||||||||
| ≤10 years | 79 | 1.0 | 1.0 | 58 | 1.0 | 1.0 | ||||||||
| >10 years | 52 | 1.21 | 0.67–2.2 | 0.52 | 1.15 | 0.58–2.97 | 0.68 | 86 | 0.99 | 0.63–1.56 | 0.99 | 1.09 | 0.67–1.77 | 0.73 |
| Tumor size | ||||||||||||||
| ≤5 cm | 66 | 1.0 | 1.0 | 35 | 1.0 | 1.0 | ||||||||
| >5 cm | 65 | 1.45 | 0.49–1.74 | 0.80 | 1.06 | 0.51–2.17 | 0.89 | 109 | 0.71 | 0.44–1.15 | 0.17 | 0.74 | 0.45–1.20 | 0.22 |
| IRS | ||||||||||||||
| I + II | 21 | 1.0 | 1.0 | |||||||||||
| III | 110 | 1.44 | 0.53–3.96 | 0.47 | 1.60 | 0.45–5.73 | 0.47 | |||||||
| T-status | ||||||||||||||
| T1 | 68 | 1.0 | 1.0 | 16 | 1.0 | 1.0 | ||||||||
| T2 | 63 | 1.18 | 0.62–2.25 | 0.62 | 1.22 | 0.58–2.56 | 0.60 | 128 | 1.22 | 0.61–2.45 | 0.57 | 1.82 | 0.83–4.01 | 0.14 |
| N-status | ||||||||||||||
| N0 | 83 | 1.0 | 1.0 | 13 | 1.0 | 1.0 | ||||||||
| N1 | 48 | 1.43 | 0.76–2.67 | 0.26 | 1.60 | 0.78–3.27 | 0.20 | 111 | 0.77 | 0.49–1.20 | 0.25 | 0.87 | 0.55–1.40 | 0.57 |
| B/BM | ||||||||||||||
| Yes | 93 | 1.0 | 1.0 | |||||||||||
| No | 51 | 0.61 | 0.39–0.97 | 0.04 | 0.66 | 0.41–1.09 | 0.09 | |||||||
| Fusion | ||||||||||||||
| Negative | 17 | 1.0 | 1.0 | 13 | 1.0 | 1.0 | ||||||||
| PAX3 pos | 74 | 9.34 | 1.29–69.1 | 0.03 | 6.52 | 0.88–48.5 | 0.07 | 95 | 5.33 | 1.89–15.1 | 0.002 | 4.41 | 1.57–12.5 | 0.005 |
| PAX7 pos | 22 | 7.29 | 0.89–59.5 | 0.06 | 4.19 | 0.49–36.0 | 0.19 | 16 | 2.67 | 0.85–8.36 | 0.09 | 2.10 | 0.65–6.81 | 0.21 |
| PAX not known | 18 | 3.58 | 0.37–34.7 | 0.27 | 2.34 | 0.21–26.1 | 0.49 | 20 | 3.09 | 0.86–8.36 | 0.06 | 2.59 | 0.79–8.53 | 0.12 |
- Note: PAX3 vs.−PAX7 HR EFS p = 0.55, HR OS p = 0.37 and PAX3 vs.- PAX7 HR EFS 0.04, pHR OS 0.04.
- a Number of events for EFS 50, for OS 37.
- b Number of events for EFS 113, for OS 104.
Among patients with metastatic disease, patients aged >10 years, with FP tumors, PAX3::FOXO1 fusion type and B/BM involvement, had significantly worse EFS and OS compared to patients aged ≤10 years, without B/BM involvement, FN tumors, or with PAX7::FOXO1 fusion type (Table 3). Tumor invasiveness (T2) was adversely associated with OS only. Multivariate analysis in patients with metastatic tumors, identified PAX3::FOXO1 fusion as an independent adverse prognostic factor for EFS and OS, B/BM involvement for EFS only (Table 4).
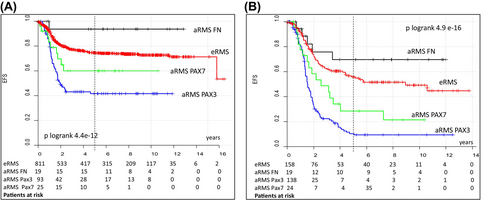
When comparing the outcomes of patients with FN-, PAX3:: or PAX7::FOXO1-positive localized aRMS to those with eRMS treated in the CWS trials included in this analysis (Figure 2, Table S6), patients with PAX3::FOXO1-positive tumors had significantly worse EFS and OS. Patients with FN aRMS had better EFS and OS without reaching statistical significance (5 year EFS 94% vs. 74%, 5 year OS 94%vs. 83%) and the results for patients with PAX7::FOXO1-positive tumors were comparable to those for eRMS (5 year EFS 60% vs. 74%, 5 year OS 80%vs. 83%). In patients with metastatic tumors, PAX3::FOXO1 and PAX7::FOXO1-positive aRMS had significantly worse EFS and OS compared to patients with eRMS in contrast to patients with FN aRMS whose EFS and OS were much better but did not reach statistical significance (5 year EFS 71% vs. 45%, 5 year OS 70%vs. 56%).
4 DISCUSSION
The present analysis describes the survival outcomes in a large group of patients with localized and metastatic aRMS, prospectively enrolled and uniformly treated in recent CWS trials and registry, in relation to FOXO1 fusion status and fusion type (PAX3 vs. PAX7) in the context of known clinical factors associated with prognosis. The percentage of FN aRMS in our analysis was lower (10%) than in other published series which reported 16%–38%, probably due to the differences in the diagnostic criteria for aRMS.12, 20, 21, 23, 32, 33 The International Classification of RMS (ICR) published in 1995 modified the diagnostic criteria for aRMS.34 This change resulted in an increase in the frequency of aRMS and doubled the proportion of FOXO1 fusion negative aRMS cases to 37% on D9803.23 Upon re-review using current diagnostic criteria the percentage of FN aRMS decreased to 18%. The proportion of FN aRMS in the analysis of patients treated in MMT trials in the UK was also much higher at 20/54 (38%).32 All of these differences and changes in diagnostic criteria may affect not only the incidence of aRMS, but likely also the incidence, composition, and prognosis of FN aRMS. In the CWS studies, which included immunohistomorphology early in their classification system, the introduction of ICR did not result in major changes and the proportion of aRMS remains constant at around 15%–20% with FN aRMS of 6%–11%.2, 4, 6, 35 The FOXO1 fusion negative aRMS has been described as a molecularly heterogeneous group comprising cases with rare fusion-positive cells, cases with alternate rare genetic fusions, cases with genomic fusions and no detectable RNA product and cases with no evidence of molecular fusion.30, 36
Because patients with localized and metastatic RMS have different distributions of histologic subtypes, clinical characteristics and prognosis, we analyzed and discussed these two groups separately.
Interestingly, we found no differences in the distribution of clinical features between FP- and FN-localized aRMS. When comparing localized PAX3:: vs. PAX7::FOXO1 positive tumors, the only difference was the significantly higher percentage of less invasive (T1) tumors in the PAX7::FOXO1 positive group. Williamson et al. reported that the proportion of tumors occurring in favorable sites is significantly lower in FP than in FN aRMS, but patients with localized and metastatic disease were analyzed together.20 An association between a higher rate of PAX7::FOXO1 fusion and low-risk clinical features was found in the analyses of COG D9602 and D9803.21, 37 Differences in the composition of the cohorts studied may limit comparability with our results. Despite similarities in the distribution of clinical factors between patients with FP and FN localized tumors in our cohort, fusion status together with IRS group and lymph node status were significantly associated with EFS and OS on univariate analysis. Tumor size, invasiveness, and PAX variant were predictive for OS. Patients with PAX7::FOXO1 positive tumors had a significantly better OS compared to those with PAX3::FOXO1 positive tumors (81% vs. 50%, p = 0.03), slightly worse compared to FN (81% vs. 94%) and similar to those with eRMS (81% vs. 84%). Multivariate analysis showed that only PAX3::FOXO1 fusion was an independent adverse prognostic factor for EFS in patients with localized tumors.
Many studies have identified FOXO1 fusion status as a prognostic factor in RMS and have also reported on the additional impact of clinical factors, but most have analyzed localized and metastatic tumors together and been conducted with convenience cohorts.16, 19, 20, 38, 39 Few have analyzed the impact of fusion status and other prognostic factors in patients with localized RMS treated in prospective trials and differentiated between FN eRMS and FN aRMS.21, 22, 24, 33, 37, 40 They found that the prognostic significance of fusion status and its type depends on other clinical factors and study cohorts. In the COG and EpSSG analysis of patients with aRMS and lymph node involvement (N1), EFS for patients with FOXO1-positive tumors treated on EpSSG RMS 2005 was significantly inferior to those FOXO1-negative. Tumor invasiveness (T) was identified as an additional independent prognostic factor. In contrast, in the COG trial ARST0531, the EFS of patients with FOXO1-positive and negative N1 aRMS was similar.40
An analysis of the COG D9803 trial showed that OS was significantly inferior for patients with PAX3::FOXO1 positive RMS compared to the PAX7::FOXO1positive group, whose OS was comparable to that of patients with FN aRMS and eRMS, in line with our results. However, the significance of PAX variant for OS decreases when analyzed in patients with favorable stage and IRS group.21
In the analysis of patients with FP localized aRMS treated on three COG studies (D9602, D9803 and ARST0531) an association with EFS was found for patient age, tumor size and invasiveness. Age, IRS group, tumor size, site and invasiveness, nodal status and fusion type (PAX7:: vs. PAX3::FOXO1) were significantly associated with OS.24 It is intriguing that in our and COG analysis there was a large overlap in clinical factors associated with OS (IRS group, tumor size and invasiveness, nodal status and fusion type PAX3:: vs. PAX7::FOXO1) but different factors were associated with EFS (COG: age, tumor size and invasiveness, CWS: IRS group and nodal status). Surprisingly, in the COG analysis lymph node involvement (N0 vs. N1) had no impact on EFS (4-year EFS 56% vs. 47%). The authors suggested that tumor size may contribute more than lymph node involvement in predicting the likelihood of relapse in patients with localized FP RMS. Multivariate analysis of the COG cohort identified older age (≥10 years) and tumor size >5 cm as independent adverse prognostic factors for EFS, and for OS in addition to tumor size, tumor invasiveness (T2) and PAX3::FOXO1 variant. The correlation of PAX3::FOXO1 with inferior OS decreased; however, when associated with other adverse prognostic factors such as large tumor size and older age.24 Despite differences in the prognostic significance of PAX3::FOXO1 versus PAX7::FOXO1 fusion for EFS and OS between the two COG analyses and ours, there is a consensus that PAX3::FOXO1 fusion is associated with poor prognosis rather than PAX7::FOXO1. The prognostic significance of PAX variants has also been previously reported based on convenience cohorts.19, 41, 42 However, differences in prognosis between patients with PAX7 and PAX3 fusion positive tumors have not been recognized as sufficiently proven to be incorporated into current risk stratification. Many molecular differences that support the clinical differences have been reported. PAX7::FOXO1 fusion gene is usually amplified while the PAX3::FOXO1 fusion gene is rarely amplified.43
It has been shown that the PAX3::FOXO1- and PAX7::FOXO1 positive subsets are molecularly distinguished by DNA methylation suggesting that epigenetic differences may contribute to the distinct biology and clinical features between these two FP subsets.44
Manceau et al. showed that PAX3::FOXO1 and PAX7::FOXO1 generate partially divergent transcriptomic signatures, which include genes encoding regulators of cell morphology and cell cycle and demonstrated a differential mode of action between the two chimeric proteins that could in turn support the clinical differences.45
In contrast to patients with localized tumors, the distribution of clinical characteristics differed between patients with FP and FN metastatic aRMS. The number of patients older than 10 years, with unfavorable tumor sites, and B/BM metastases was significantly higher in the FP cohort. Age was the only factor with a significantly different distribution between patients with PAX3:: vs. PAX7::FOXO1 positive tumors. Sixty-seven percentage of patients with PAX3::FOXO1 positive aRMS were older than 10 years. In univariate analysis, age, B/BM metastases, fusion status and PAX variant were significantly associated with EFS and OS, T status with OS only; PAX3::FOXO1 fusion remained relevant for EFS and OS in multivariate analysis, B/BM for EFS only.
Although the prognostic role of FOXO1 fusion status is widely accepted in localized disease, it is still controversial in metastatic disease. Rudzinski et al. examined outcome depending on histology and fusion status in 178 patients with metastatic RMS (78% being diagnosed as aRMS) on COG D9802 and ARST0431.23 This study revealed that clinical risk factors have the most impact on outcome. Fusion status being more common in patients with higher Oberlin score but was not an independent biomarker. In contrast to our results, no significant difference was found between PAX3::FOXO1, PAX7::FOXO1 and FN cases when analyzing tumors with exclusively alveolar histology.23 The reason for the difference in the significance of fusion status between our report and COG's may be the much lower 5-year EFS for patients with FN aRMS (COG 29% vs. 71% in our analysis). In another COG analysis of localized and metastatic patients treated on six trials, FOXO1 fusion emerged as the strongest prognostic variable, with no clinical factor further subdividing outcome in patients with metastatic FP tumors.22 However, this analysis did not distinguish between PAX3::FOXO1 versus PAX7::FOXO1 and FN aRMS versus eRMS. Although PAX3::FOXO1 fusion itself has prognostic value, no molecular markers are currently available for risk stratification of FP tumors. CDK4 and MYCN amplifications have been observed in FP aRMS and appear to be associated with prognosis but future studies are needed to validate their significance.46 Secondary objectives of the ongoing COG ARST2031 (NCT049941329) include evaluating the impact of copy number variation, including CDK4 and MYC on outcome, which should lead to the clarification of the prognostic value of these markers. Similarly, MIR17HG may have prognostic value in PAX7::FOXO1 positive aRMS.47
A limitation of our study is the fact that 22% of the tumors were not tested for fusion status. However, this is comparable to the EpSSG analysis (19%), lower than in the COG analysis for metastatic patients (37%), but higher than the COG cohort of patients with N1 positive RMS where only 7% were not tested.23, 33, 40
- PAX3::FOXO1 fusion status emerged as an independent predictor of poor EFS in patients with localized aRMS. On univariate analysis, clinical factors such as IRS group III and lymph node involvement were predictive of inferior EFS and OS, tumor size >5 cm, invasiveness (T2) for inferior OS.
- In patients with metastatic disease, PAX3::FOXO1 fusion was the independent indicator of poor EFS and OS, and B/BM metastases for poor EFS. In univariate analysis, age >10 years was additionally associated with worse EFS and OS.
- The proportion of FN aRMS was very low (10%) compared to other published series, highlighting the difficulty of considering this group well defined and comparable between studies. They had a remarkably favorable prognosis of 5-year EFS of 94% for localized and 71% for metastatic disease, which is better than for PAX7::FOXO1 tumors and eRMS.
- PAX7::FOXO1-positive aRMS were associated with significantly better outcome (EFS and OS in metastatic and OS in localized disease) than PAX3::FOXO1-positive aRMS (Tables 2 and 3) and with comparable outcome (EFS and OS) to eRMS in localized disease (Table S6).
Our analysis of prospectively treated patients supports the finding of Missiaglia et al. that only PAX3::FOXO1 fusion is an independent adverse prognostic factor in patients with localized and metastatic aRMS and should replace FOXO1 fusion in risk stratification. PAX7::FOXO1 and FN aRMS represent subgroups with distinct prognostic relevance. Clinical factors with prognostic relevance described in this report should be considered in the risk stratification of patients with aRMS.
However, the next step should be the integration of molecular features beyond FOXO1 fusion status into risk stratification and therapeutic decisions. Ongoing COG, CWS, and EpSSG trials and registries have included molecular characterization as accompanying studies. The results of these studies will hopefully provide the basis for new, more precise risk stratification of patients with RMS.
AUTHOR CONTRIBUTIONS
Ewa Koscielniak: Conceptualization (lead); formal analysis (lead); funding acquisition (lead); methodology (lead); project administration (lead); supervision (lead); writing – original draft (lead); writing – review and editing (lead). Sabine Stegmaier: Conceptualization (equal); formal analysis (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Gustaf Ljungman: Formal analysis (supporting); writing – review and editing (equal). Bernarda Kazanowska: Investigation (equal); writing – review and editing (equal). Felix Niggli: Resources (equal); writing – review and editing (equal). Ruth Ladenstein: Resources (equal); writing – review and editing (equal). Bernd Blank: Data curation (equal); formal analysis (equal); software (equal); visualization (equal); writing – review and editing (equal). Erika Hallmen: Data curation (equal); formal analysis (equal); methodology (equal); software (equal); validation (equal); writing – review and editing (equal). Christian Vokuhl: Conceptualization (equal); methodology (equal); resources (equal); writing – review and editing (equal). Claudia Blattmann: Resources (equal); writing – review and editing (equal). Monika Sparber-Sauer: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Thomas Klingebiel: Conceptualization (equal); funding acquisition (equal); methodology (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
We thank all patients, parents, national and international investigators and data managers who participated in the CWS-studies, and Dr. Mary Morgan, a native speaking colleague, and former study assistant of the CWS for review of the manuscript. We are especially indebted to Prof. Ivo Leuschner, a longtime reference pathologist of the CWS, who unexpectedly passed away in 2019, for his fundamental contribution to improving the diagnostics and classification of rhabdomyosarcoma.
FUNDING INFORMATION
The CWS-2002P and CWS-IV 2002 trials were supported by grants from the German Cancer Aid Foundation. (CWS-2002P: 50-2721-Tr2. CWS-IV 2002: 50-2695), the CWS Registry SoTiSaR from the German Children's Cancer Foundation (DKS 2009.08. 2012.04 and 2015.12) and by the Förderkreis Krebskranke Kinder Stuttgart. Germany (continuous support).
CONFLICT OF INTEREST STATEMENT
The authors have no potential conflicts of interest related to any aspect of this analysis.
Open Research
DATA AVAILABILITY STATEMENT
Individual participant data are not publicly available as this was not a requirement of the study and registry protocols.