A highly sensitive screening system to evaluate the reversibility of neuroendocrine prostate cancer to prostate adenocarcinoma
Abstract
Background
Lineage plasticity in prostate cancer primarily induces transdifferentiation of adenocarcinoma into neuroendocrine prostate cancer (NEPC). Lineage alteration is largely driven by epigenome and is potentially reversible. Nonetheless, evidence supporting NEPC reversibility is lacking in materials derived from clinical specimens. Hence, we established KUCaP13, a novel NEPC cell line derived from the tissue of a patient initially diagnosed with adenocarcinoma, which later recurred as NEPC. We aimed to prove the reversibility of cellular lineage by high-throughput chemical screening using KUCaP13 cells.
Methods
Compounds responsible for androgen receptor (AR) re-expression in KUCaP13 cells were screened. A reporter gene, androgen response element luciferase (AREluc), was transduced into KUCaP13 cells to detect AR activity using luciferase assay.
Results
Positive control cells (KUCaP13_AREluc_AR) overexpressing AR showed enhanced luminescence upon administration of synthetic androgen. An initial screen of 1552 compounds revealed 30 candidate molecules potentially enhancing luciferase luminescence. In the second screening, we eliminated false positives and validated the findings using luciferase assay and quantitative real-time polymerase chain reaction. However, all hit compounds were confirmed as false positives, probably due to their inhibitory activity on luciferase.
Conclusions
We developed a highly sensitive screening system to evaluate the reversibility of plasticity in NEPC using a novel cell line. However, no compound demonstrated the ability to re-express AR in this cell line. Nevertheless, in future studies, this screening system could prove valuable for elucidating the mechanism of lineage plasticity in NEPC and for developing novel therapies focused on reversing plasticity.
1 INTRODUCTION
Prostate cancer grows in an androgen-dependent manner, and the standard therapy for advanced prostate cancer is endocrine therapy targeting the androgen receptor (AR) signaling pathway. However, with the widespread use of potent next-generation AR signaling inhibitors (ARSIs), the incidence of treatment-related neuroendocrine prostate cancer (t-NEPC), which is completely independent of the AR pathway, is rapidly rising.1, 2 Unlike its AR-dependent ancestor, t-NEPC remains a poor prognosis cancer, lacking effective treatment options, and thus necessitating the exploration and development of innovative therapies.2
Transdifferentiation of prostate adenocarcinoma into neuroendocrine prostate cancer (NEPC) occurs through lineage plasticity.3-5 Lineage plasticity is a biological process that enhances cell survival by enabling adaptation to the environment, avoidance of stress, or tissue repair.4 Within cancer, lineage plasticity facilitates the development of therapy resistance in cancer cells by reprogramming into therapy-resistant phenotypes that bypass targeted therapies.5 This phenomenon is especially notable in cancer varieties where there are potent targeted therapies for key growth pathways, such as AR-driven prostate cancer, epidermal growth factor receptor (EGFR)-mutant lung cancer, and BRAF-mutant melanoma.4
Recent genomic analyses have revealed several alterations enriched in t-NEPC, with the loss of tumor suppressor genes (TP53 and RB1) being crucial genomic changes linked to t-NEPC.3, 6 Moreover, epigenetic genes, such as EZH2 and SOX2, induce neuroendocrine (NE) transdifferentiation.7, 8 A study with gene-engineered mice lacking Pten and Rb1, or all three (Pten, Rb1, and Trp53), showed reduced expression of AR and increased expression of NE-related genes, phenocopying human NEPC. Furthermore, using EZH2 inhibitors restored AR expression and sensitivity to antiandrogen therapy.7 These findings suggest that plasticity in t-NEPC is potentially reversible, and regulating cellular lineage could serve as a novel therapeutic strategy. However, EZH2 inhibition did not increase AR expression or activity in organoids from human NEPC.9 To date, the reversibility of lineage plasticity in prostate cancer has not been confirmed in human-derived t-NEPC clinical models.
One major hurdle in NEPC research is the paucity of human-derived t-NEPC cell lines suitable for genetic manipulation or large-scale compound screening. Previously, we created a novel t-NEPC cell line called KUCaP13, derived from a patient-derived xenograft (PDX) and verified its lineage originating from prostate adenocarcinoma.10 The cell line's origin as prostate adenocarcinoma is supported by the preservation of a homozygous CHD1 deletion from patient tissue to the cell line.10 Concurrent inactivation of TP53, RB1, and PTEN in KUCaP13 makes it an ideal candidate for NEPC research.11 KUCaP13 cells grow suspended in culture, forming spheroids. They are dissociated into single cells to facilitate lentiviral transfection, fluorescence-activated cell sorting (FACS) sorting, and accurate cell counting. Herein, we hypothesized that t-NEPC can be transdifferentiated back to adenocarcinoma and attempted to prove the reversibility of cellular lineage by compound screening using KUCaP13 cells (Figure S1). To this end, we developed a highly sensitive screening system to detect AR re-expression in KUCaP13 cells and performed a high-throughput screening using a chemical library.
2 MATERIALS AND METHODS
2.1 Cell culture
The LNCaP cell line was purchased from the American Type Culture Collection (Rockville, MD, USA). KUCaP13 and LNCaP cells were cultured in RPMI 1640 medium (Nacalai Tesque, Kyoto, Japan; Cat no. 30264-56) supplemented with 10% fetal bovine serum (Merck Millipore, Darmstadt, Germany), 12.5 mmol/L HEPES, penicillin, and streptomycin, at 37°C in 5% CO2. For experiments investigating AR activity and expression, the cells were grown in an androgen-free medium composed of phenol red-free RPMI 1640 (Thermo Fisher Scientific, Waltham, MA, USA; Cat no. 11835030) containing 10% charcoal-stripped fetal bovine serum (Cytiva, Tokyo, Japan; Cat no. SH30068.03) and 12.5 mmol/L HEPES, with or without R1881 (Merck Millipore).
2.2 Lentiviral transduction of cell lines
Androgen response elements (AREs) with luciferase reporter was introduced into LNCaP cells to generate LNCaP_AREluc cells using AR-Luciferase (Puro) lentiviral particles (LVP914-P) (GenTarget Inc, San Diego, CA, USA). As a negative control, LNCaP was infected with miniPro (Null)-Luc (Puro) lentiviral particles (Path-Ctr3) (GenTarget Inc) to generate LNCaP_miniluc cells using polybrene. The infected cells were then selected using puromycin (1.0 μg/mL).
To facilitate transduction with lentivirus, KUCaP13 cells were dissociated into single cells using TrypLE™ Express (Thermo Fisher Scientific) for 10 min at 37°C on a shaking platform (1200 rpm). Further, KUCaP13 cells were infected with AR-Luciferase (GFP) lentiviral particles (LVP914-G) (GenTarget Inc) using LentiBlast Premium (OZ Biosciences, Marseille, France) to generate KUCaP13_AREluc cells. GFP-positive cells were isolated utilizing BD FACS AriaTM II (BD Biosciences, San Jose, CA, USA) and fluorescence images were captured with a BZ-8000 microscope (KEYENCE, Osaka, Japan). Plasmid pLENTI6.3/AR-GC-E2325 (Plasmid #85128) was purchased from Addgene (Watertown, MA, USA) for AR overexpression, and viral particles were produced as described previously.10 KUCaP13_AREluc cells were infected with the viral particles to generate KUCaP13_AREluc_AR cells, which overexpresses AR. The infected cells were subjected to selection with blasticidin (4.0 μg/mL).
2.3 Western blotting
We performed protein extraction from cell pellets and measured protein concentration as previously described.10 Samples containing 12.5 μg of protein underwent loading, separation through sodium dodecyl-sulfate polyacrylamide gel electrophoresis, and subsequent transfer onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P PVDF 0.45 μm, Merck Millipore; Cat no. IPVH304F0) using a Mini Trans-Blot Cell system (Bio-Rad Laboratories, Hercules, CA, USA). After blocking in 5% milk in TBST buffer for 1 h at room temperature, the membrane was subsequently incubated overnight at 4°C with primary antibodies against AR (Cat no. 5153) and α-tubulin (Cat no. 2144), both sourced from Cell Signaling Technology (Danvers, MA, USA). Following three washes with 1 × TBST buffer, the membrane was incubated with a secondary antibody for 1 h at room temperature. Protein bands were then detected using Pierce™ ECL Western Blotting Substrate (Thermo Fisher Scientific. Cat no. 32106) and photographed with ImageQuant LAS 4000 mini (Fuji Film, Tokyo, Japan).
2.4 Luminescence measurements
Luciferase assays were performed in white 96-well plates seeded with AREluc-transfected cells. The firefly luciferase activities were quantified using a ONE-Glo™ EX Luciferase Assay System (Promega, Madison, WI, USA). Luminescence was measured using an ARVO X5 (PerkinElmer, Waltham, MA, USA) luminometer.
2.5 Chemical library
The chemical library used for chemical screening were provided by the Medical Research Support Center, Graduate School of Medicine, Kyoto University. The chemical library comprised of 1552 known compounds mainly sourced from Prestwick Chemical (Illkirch-Graffenstaden, France), Calbiochem (Merck Millipore), and Selleck Chemicals (Houston, TX, USA). Each compound are resuspended with dimethyl sulfoxide (DMSO) at a concentration of 10 mM, and placed into 96-well microtiter plates (see Figure 2A for detailed position).
2.6 Quantitative real-time polymerase chain reaction (qPCR)
Total RNA extraction was conducted with the RNeasy Mini Kit (Qiagen, Hilden, Germany), followed by reverse transcription to complementary DNA using the ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan) in accordance with the manufacturer's guidelines. Triplicate qPCR experiments were performed using PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific) on an Applied Biosystems 7300 qPCR system (Thermo Fisher Scientific). Thermal cycling parameters consisted of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. Data normalization was achieved by comparing cycle threshold values to the amplified levels of human glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
The PCR primers used were as follows: AR, F: 5′-CTTCACCAATGTCAACTCCA-3′, R: 5′-TCATTCGGACACACTGGCTG-3′; KLK3, F: 5′-CACAGCCTGTTTCATCCTGA-3′, R: 5′-AGGTCCATGACCTTCACAGC-3′; GAPDH, F: 5′-GAA GGT GAA GGT CGG AGT-3′, and R: 5′-GAA GAT GGT GAT GGG ATT TC-3′.
A GSK-3β inhibitor AR-A014418 (Cat no. S7435), an IKK inhibitor BMS-345541 (Cat no. S8044), and a JAK1/JAK2 inhibitor Momelotinib (CYT387, Cat no. S2219) were purchased from Selleck Chemicals.
2.7 Statistical analyses
Mean values along with standard error of the mean (SEM) or standard deviation (SD) are provided for the results. Student's t-test was employed to assess differences between means, with statistical significance defined as a p-value below 0.05. Z'-factor values, a measure of assay robustness, were calculated as described previously: Z'-factor = 1−(3αp + 3αn)/|βp−βn|, where αp and αn denote the standard deviations of positive and negative controls, respectively, and βp and βn represent the means of positive and negative controls, respectively.12 Statistical analyses were conducted using GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA).
3 RESULTS
3.1 Application of the AREluc reporter assay in KUCaP13 cells
We developed an AREluc luciferase reporter assay system to detect AR activity using lentivirus. The virus carried a luciferase reporter under a minimal CMV promoter (mCMV) embedded with optimized tandem repeats of the ARE sequence motif (5′- TGGAGGAACATATTGTATTTATT) and contained a constitutively expressed GFP selection marker or a puromycin selection marker under the RSV promoter (Figure 1A). When methyltrienolone (R1881), a synthetic androgen agonist, binds to AR, the activated AR then binds to ARE, inducing downstream luciferase expression. The luminescence emitted by luciferase can be easily and rapidly quantified using a luciferase assay. Conversely, AR-expressing cells, in the absence of R1881, fail to bind to ARE and consequently do not exhibit luciferase expression. Similarly, cells lacking AR expression, despite R1881 addition, do not express luciferase (Figure 1B).
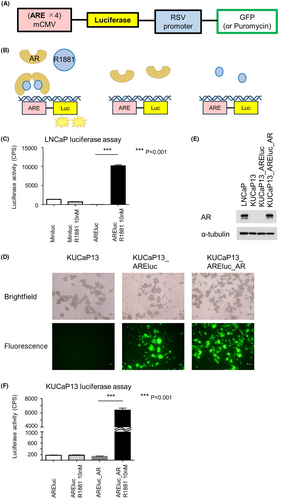
The AR-expressing prostate cancer cell line, LNCaP, was used to assess AREluc reporter assay functionality. Luminescence was significantly increased in LNCaP_AREluc cells when exposed to R1881 compared with in R1881 absence. In contrast, no such enhancement was observed in LNCaP_miniluc cells (serving as the negative control) upon the addition of R1881 (Figure 1C). These results confirm the AREluc assay's functionality in AR-expressing cells.
Furthermore, KUCaP13 cells were transduced with the AREluc reporter (containing a GFP selection marker) to generate KUCaP13_ARE-luc cells. Since KUCaP13 cells lack AR expression, KUCaP13_AREluc_AR cells with forced AR expression served as the positive control in this assay system. GFP signals were detected in both KUCaP13_AREluc cells and KUCaP13_AREluc_AR cells using a fluorescence microscope, confirming successful AREluc transduction (Figure 1D). Western blotting confirmed the expression of AR in KUCaP13_AREluc_AR cells (Figure 1E).
Finally, we validated the functionality of the AREluc reporter assay in KUCaP13 cells. Here, KUCaP13_AREluc_AR cells' luminescence exhibited a significant increase upon exposure to R1881 compared with its absence, whereas no such augmentation was observed in KUCaP13_AREluc cells regardless of the presence or absence of R1881 (Figure 1F). The Z' factor, a quality control parameter calculated for the AREluc reporter assay in KUCaP13, was 0.81, indicating the high performance of the assay.12 These results demonstrate the AREluc assay's proper functionality in KUCaP13 cells and its strong applicability in high-throughput screening.
3.2 High-throughput screening using a chemical library
We performed a high-throughput screening with KUCaP13_AREluc cells using a chemical library, as illustrated in Figure 2A. KUCaP13_AREluc and KUCaP13_AREluc_AR cells were dissociated into single cells using TripLE™ and subsequently seeded (5.0 × 104 cells/80 μL) in white 96-well plates of androgen-free medium with or without 10 nM R1881 (Figure 2B), and incubated for 24 h at 37°C. Individual compound solutions (diluted 1:200 in androgen-free medium) were added (20 μL/well), resulting in a final concentration of 10 μM per compound. After 48 h incubation at 37°C, we added 100 μL of luciferase assay reagent and measured the luminescence. Duplicate plates were tested for each chemical library plate.
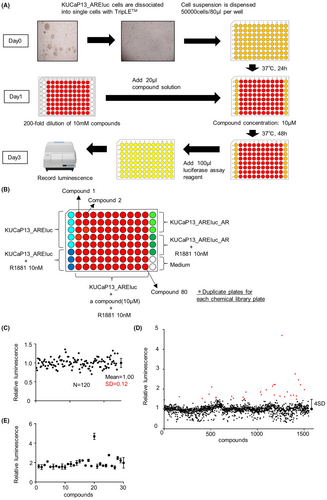
The first screening was performed for 1552 compounds of the chemical library by 40 independent measurements using 96-well plates. The mean ± SD of Z' factors in the first screening was 0.87 ± 0.06. In each plate, the relative luminescence of each well was calculated and normalized by the mean luminescence from the wells of KUCaP13_AREluc cells without R1881 on the same plate. The mean relative luminescence values of the wells treated with each compound are listed in Table S1. The SD of the relative luminescence was calculated from the wells of KUCaP13_AREluc cells treated with 10 nM R1881 without any compound, resulting in a value of 0.12 (Figure 2C). We set the cutoff of the relative luminescence value for a significant signal as 1 + 4SD (1.48), identifying 30 hit compounds (Figure 2D). All the hit compounds and their individual results in the first screening are listed in Table 1. Additionally, the scatter plot extracting the relative luminescence of the hit compounds is shown in Figure 2E.
| Compound | Relative luminescence | Class | |
|---|---|---|---|
| Mean | SD | ||
| AR-A014418 | 4.70 | 0.23 | GSK3β inhibitor |
| BMS-345541 | 2.77 | 0.06 | IkB kinase inhibitor |
| MK-571 sodium salt hydrate | 2.47 | 0.12 | Leukotriene receptor antagonist |
| Cyt387 | 2.23 | 0.09 | Janus kinase inhibitor |
| A-966492 | 2.22 | 0.04 | PARP inhibitor |
| PD 146176 | 2.22 | 0.17 | Lipoxygenase inhibitor |
| Crenolanib | 2.15 | 0.13 | FLT/PDGFR inhibitor |
| Tiabendazole | 2.11 | 0.06 | Anthelmintic |
| BMS 493 | 1.98 | 0.38 | Inverse pan-retinoic acid receptor agonist |
| Anethole-trithione | 1.97 | 0.18 | Bile secretion-stimulating agent |
| MTEP hydrochloride | 1.86 | 0.10 | Metabotropic glutamate receptor antagonist |
| Lansoprazole | 1.85 | 0.18 | Proton pump inhibitor |
| Nabumetone | 1.85 | 0.05 | NSAID |
| Tiaprofenic acid | 1.75 | 0.03 | NSAID |
| Methiazole | 1.72 | 0.07 | Anthelmintic |
| OSI-420 | 1.70 | 0.08 | Metabolite of erlotinib |
| PF-573228 | 1.70 | 0.10 | Focal adhesion kinase inhibitor |
| Safinamide mesylate salt | 1.69 | 0.01 | Monoamine oxidase inhibitor |
| Biphenyl-indanone A | 1.66 | 0.01 | Metabotropic glutamate receptor potentiator |
| Fenobam | 1.65 | 0.02 | Metabotropic glutamate receptor antagonist |
| Demeclocycline hydrochloride | 1.64 | 0.05 | Antibiotic |
| Riluzole hydrochloride | 1.62 | 0.12 | GABA uptake inhibitor |
| SB 204741 | 1.62 | 0.16 | Serotonin receptor antagonist |
| Rabeprazole Sodium salt | 1.59 | 0.19 | Proton pump inhibitor |
| Tenatoprazole | 1.59 | 0.18 | Proton pump inhibitor |
| Dacarbazine | 1.57 | 0.08 | Antineoplastic alkylating agent |
| Leflunomide | 1.54 | 0.02 | DMARD |
| Erlotinib | 1.53 | 0.18 | EGFR inhibitor |
| Butylparaben | 1.52 | 0.15 | Antimicrobial agent |
| LY255582 | 1.50 | 0.12 | Opioid receptor antagonist |
- Abbreviations: DMARD, disease-modifying antirheumatic drug; EGFR, epidermal growth factor receptor; FLT, FMS-like tyrosine kinase; GABA, gamma-aminobutyric acid; GSK3β, glycogen synthase kinase 3β; NSAID, non-steroidal anti-inflammatory drug; PARP, poly ADP ribose polymerase; PDGFR, platelet-derived growth factor receptor.
3.3 Validation of hit compounds
We conducted a second screening using luciferase assay to validate the 30 hit compounds and eliminate false positives. False positives were detected by comparing the plates with and without R1881 added to the wells containing each hit compound. The screening was performed in duplicates. The second screening was performed in the same manner as the first screening (Figure 2A). As shown in Figure 3A, in a 96-well plate, eight compounds were initially diluted to 50 μM and further diluted in a threefold serial manner to prepare a dilution series. Then, 20 μL of each compound solution was added to the 96-well white plates with seeded cells, resulting in a 3-fold serial dilution series starting at a final concentration of 10 μM for each of the eight compounds.
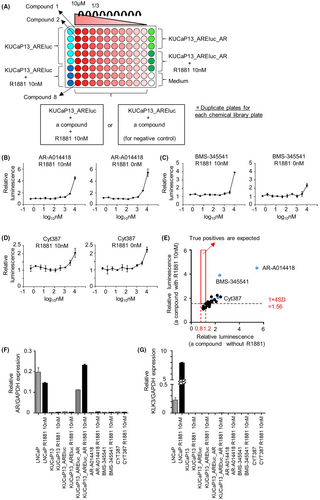
The second screening was performed for the 30 hit compounds by 16 individual measurements using 96-well plates. The mean ± SD of Z' factors in the second screening was 0.81 ± 0.09. The results of each plate were standardized in the same manner as in the first screening. The results of the second screening are shown in Figure 3B–D and Figure S2. For all candidate compounds, the increase in relative luminescence was observed with or without R1881 addition, exhibiting similar dose–response curves. The relative luminescence cutoff value from wells with R1881 addition was determined to be 1 + 4 SD (1.56), using a similar calculation as in the first screening. The relative luminescence from wells without R1881 addition (serving as the negative control) was expected to fall within the range of 0.8–1.2 if there was no signal. Notably, none of the compounds met both criteria (increased luminescence only with R1881 and control range without R1881) for true positives (Figure 3E).
We also checked the mRNA expression of AR and its downstream target KLK3 induced by the hit compounds for three representative candidate compounds: AR-A014418 (a GSK-3β inhibitor), BMS-345541 (an IKK inhibitor), and CYT387 (a JAK inhibitor) using qPCR. However, these drugs did not alter the expression of AR or KLK3 (Figure 3F,G). Notably, KLK3 expression was also not induced in KUCaP13_AREluc_AR cells with overexpressed AR, suggesting that AR expression alone is not sufficient to activate the AR pathway in KUCaP13 cells. In summary, the compounds identified as candidate hits in the first screening underwent validation through luciferase assay and qPCR. However, all these compounds were considered false positives. No compound achieved AR re-expression in the KUCaP13 cells.
4 DISCUSSION
NEPC is an aggressive variant of prostate cancer. However, due to its rarity and the lack of experimental models, its biology has remained unclear. Recent advances in genomic analysis of human specimens have revealed NEPC's genomic and molecular biological characteristics. The acquisition of MYCN oncogenes, coupled with the loss of tumor suppressor genes (TP53, RB1, and PTEN), play a critical role in NEPC development.13, 14 However, since these genes are not direct therapeutic targets, NEPC remains a cancer with a very poor prognosis for which no effective treatment currently exists. The origin of NEPC, whether it arises from a small subset of pre-existing NE cells in the prostate gland or from the transdifferentiation of adenocarcinoma cells, has sparked controversy.15, 16 However, recent reports strongly suggest that t-NEPC undergoes transdifferentiation from an adenocarcinoma due to epithelial plasticity.6, 17, 18 The mechanism underlying lineage plasticity in t-NEPC is thought to be epigenetic and potentially reversible; however, the detailed processes have not been fully clarified.4
Despite recent advances in t-NEPC research, no t-NEPC cell lines are available for genetic manipulation or large-scale compound screening. The solitary widely employed cell line derived from a patient with NEPC is NCI-H660. Initially classified as originating from small-cell lung cancer, it was subsequently recognized as prostatic in nature due to the presence of TMPRSS2-ERG fusion.19 However, since the cell line originated from a lymph node metastasis in a patient who had died within a month of diagnosis without receiving treatment, NCI-H660 is likely to be a de novo NEPC, and it is uncertain whether it originally expressed AR.19, 20 Therefore, NCI-H660 is not a suitable model for studying the lineage plasticity of t-NEPC. In contrast, a novel t-NEPC cell line, named KUCaP13, was created from the tissue of a patient initially diagnosed with adenocarcinoma, which subsequently transformed into NEPC. KUCaP13 is the initial patient-derived t-NEPC cell line displaying the triple loss of tumor suppressors crucial for NEPC progression via lineage plasticity.10 In prostate cancer, lineage alteration can be evaluated by AR activity, unlike in other cancer types, such as lung cancer and melanoma. Hence, in the present study, we focused on identifying compounds that can re-express AR through chemical screening using KUCaP13 cells. Identifying these compounds will contribute to understanding the mechanism of lineage plasticity in t-NEPC and enable the development of innovative therapies for t-NEPC in combination with ARSIs.
AR is crucial in the development and progression of most prostate cancer cases. AR, a ligand-dependent transcription factor, is localized in the cytoplasm when inactive.21 Ligand binding, by the native androgens testosterone and 5α-dihydrotestosterone, induces nuclear translocation and receptor dimerization. In the nucleus, AR recruits various collaborative factors and binds ARE sequences located in the enhancer and promoter regions of target genes. Thus, it regulates the transcription of androgen-responsive genes, including prostate-specific antigens.22 Azeem et al. developed ARE-based assays that can test AR activity on a large scale, rendering them valuable for drug screening.23 Luciferase is frequently employed as a reporter in high-throughput screening assays due to its remarkable sensitivity, extensive dynamic range, and swift measurement capabilities.24 Thus, in our study, a reporter gene, AREluc, was transduced into KUCaP13 cells to detect AR activity using luciferase assay. The positive control cells, KUCaP13_AREluc overexpressing AR, exhibited enhanced luminescence upon the administration of synthetic androgens. This reporter assay system enables the assessment of plasticity reversibility by detecting AR expression in KUCaP13 and facilitates high-throughput screenings. This is a novel screening system for evaluating the reversibility of plasticity using the t-NEPC cell line.
Compounds responsible for AR re-expression in KUCaP13 cells were screened using the library of known compounds provided by our Drug Discovery Research. Previously, we identified disulfiram as a sensitizer of cisplatin in bladder cancer through high-throughput chemical screening using this library.25 Our present study detected compounds that enhance luciferase luminescence and identified 30 hit compounds in the first screening. However, all hit compounds were confirmed as false positives through the second screening and qPCR. One possible reason is the inhibitory effect of the compounds on luciferase. In firefly luciferase reporter gene assays, inhibitors of luciferase can act intracellularly to prolong the lifespan of ectopically expressed firefly luciferase enzyme. This leads to heightened luciferase activity, which can be visually indistinguishable from the activation of reporter gene transcription.26 In previous studies, inhibitory activity against luciferase was identified in 12% of the compounds present in a chemical library.27 In our present study, 73% of the compounds that exhibited false positives shared the same root scaffolds as luciferase inhibitors that had been reported previously.27
The reversibility of lineage plasticity in t-NEPC has been demonstrated in gene-engineered mice, but this has not been confirmed in models from clinical samples. Recently, the reversibility of lineage plasticity in castration-resistant prostate cancer (CRPC) in the intermediate/lineage-plastic state through the JAK/STAT pathway has been reported.28 The combination of a JAK inhibitor and an FGFR inhibitor increased AR expression in organoids with enhanced JAK/STAT signaling from patients with CRPC but did not affect AR expression in organoids from patients with NEPC.28 In the current situation, where the reversibility of cellular lineage of t-NEPC in clinical specimens has not been demonstrated, the establishment of a screening system to detect AR re-expression using t-NEPC cell lines and applying it in large-scale chemical screening may open a door for an innovative development.
In our study, a single compound alone was unable to re-express AR in KUCaP13 cells. However, since lineage plasticity involves multiple processes, there may be potential for AR re-expression in t-NEPC utilizing a synergistic combination of multiple compounds. Alternatively, it may be that the regulation of cellular lineage in cells that had undergone complete transdifferentiation to t-NEPC is challenging. In a previous study identifying the role of JAK/STAT signaling in NEPC transdifferentiation, it was reported that therapeutic timing was critical due to cell-state heterogeneity in a patient with CRPC and the absence of JAK/STAT activation in NEPC.28 Hence, reversing the cell lineage in the earlier stages of transdifferentiation may be crucial.
Our study has several limitations. This screening system was challenged by the absence of a compound that can serve as a positive control. Before compound screening, we independently tested several drugs that could potentially impact plasticity in t-NEPC, including an EZH2 inhibitor (EPZ-6438), a bromodomain inhibitor ((+)-JQ1), an LSD inhibitor (GSK2879552 2HCl), and a histone deacetylase inhibitor (Trichostatin A); however, none of them elicited an increase in luminescence in the presence of R1881 (data not shown). Therefore, we established KUCaP13_AREluc_AR cells as the positive control. Furthermore, owing to technical limitations associated with the nature of KUCaP13 cell growth in suspension and the difficulty of refreshing culture media during the compound screening process, we measured luminescence 2 days post-exposure to the compounds. In a previous report using organoids, human organoids had been exposed to compounds for 14 days to assess the reversibility of plasticity.28 In human prostate cancer-derived cells, it may take more than 2 days for AR re-expression. We set the entire screening to be completed in 3 days since luminescence in KUCaP13_AREluc_AR cells showed the most significant increase after exposure to R1881 cells for a 3-day incubation period (data not shown). Additionally, as cell viability was not assessed, it is possible that some of the compounds could have caused cell death at 10 μM, potentially resulting in false negatives.
In conclusion, we developed a highly sensitive screening system to evaluate the reversibility of plasticity in t-NEPC using KUCaP13. Despite not achieving the goal of AR-re-expression, this study paves the way for the application of high-throughput screening approaches in t-NEPC research and future exploration of alternative strategies, including targeting earlier stages of transdifferentiation or investigating synergistic combinations of compounds.
AUTHOR CONTRIBUTIONS
Tomohiro Fukui: Data curation (lead); formal analysis (lead); funding acquisition (equal); investigation (lead); methodology (equal); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (equal). Kosuke Okasho: Investigation (equal); resources (lead); writing – review and editing (equal). Yukiko Okuno: Formal analysis (equal); resources (equal); visualization (equal); writing – review and editing (equal). Maki Fujiwara: Investigation (equal). Kensuke Hikami: Investigation (equal). Arinobu Fukunaga: Investigation (equal). Takuro Sunada: Formal analysis (equal); investigation (equal). Yuki Kita: Formal analysis (equal); writing – review and editing (equal). Takayuki Sumiyoshi: Formal analysis (equal); writing – review and editing (equal). Takayuki Goto: Formal analysis (equal); writing – review and editing (equal). Ryoichi Saito: Formal analysis (equal); writing – review and editing (equal). Osamu Ogawa: Project administration (equal); supervision (equal). Takashi Kobayashi: Project administration (equal); resources (equal); supervision (equal). Shusuke Akamatsu: Conceptualization (lead); formal analysis (equal); funding acquisition (lead); methodology (equal); project administration (lead); supervision (lead); writing – review and editing (lead).
ACKNOWLEDGMENTS
We thank the colleagues and the research assistants of Department of Urology, Kyoto University Graduate School of Medicine for their support, especially Ms. Eriko Komaki. The chemical library and drug discovery research equipment were generously provided by Prof. Masatoshi Hagiwara. This research was partially supported by Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number JP23ama121034.
FUNDING INFORMATION
This research was funded by JSPS KAKENHI Grant Number JP21K19568 to Shusuke Akamatsu and by the funding from the Japanese Urological Association to Shusuke Akamatsu. Partial financial support was received from Bayer Yakuhin, Ltd to Tomohiro Fukui.
CONFLICT OF INTEREST STATEMENT
Tomohiro Fukui received research funding from Bayer Yakuhin, Ltd.
Open Research
DATA AVAILABILITY STATEMENT
Please contact the corresponding author for all the data in the current study if needed.