RP11-367G18.1 V2 enhances clear cell renal cell carcinoma progression via induction of epithelial–mesenchymal transition
I-Hung Shao, Pei-Hua Peng, Heng-Hsiung Wu, and Ji-Lin Chen contributed equally to this study.
Abstract
Purpose
Metastasis is the end stage of renal cell carcinoma (RCC), and clear cell renal cell carcinoma (ccRCC) is the most common malignant subtype. The hypoxic microenvironment is a common feature in ccRCC and plays an essential role in the regulation of epithelial–mesenchymal transition (EMT). Accumulating evidence manifests that long non-coding RNAs (lncRNAs) participate in RCC tumorigenesis and regulate hypoxia-induced EMT. Here, we identified a lncRNA RP11-367G18.1 induced by hypoxia, that was overexpressed in ccRCC tissues.
Methods
A total of 216 specimens, including 149 ccRCC tumor samples and 67 related normal kidney parenchyma tissue samples, were collected. To investigate the biological fucntions of RP11.367G18.1 in ccRCC, migration, invasion, soft agar colony formation, xenograft tumorigenicity assays, and tail vein and orthotopic metastatic mouse models were performed. The relationship between RP11-367G18.1 and downstream signaling was analyzed utilizing reporter assay, RNA pull-down, chromatin immunopreciptation, and chromatin isolation by RNA purification assays.
Results
Hypoxic conditions and overexpression of HIF-1α increased the level of RP11-367G18.1. RP11-367G18.1 induced EMT and enhanced cell migration and invasion through variant 2. Inhibition of RP11-367G18.1 variant 2 reversed hypoxia-induced EMT phenotypes. An in vivo study revealed that RP11-367G18.1 variant 2 was required for hypoxia-induced tumor growth and metastasis in ccRCC. Mechanistically, RP11-367G18.1 variant 2 interacted with p300 histone acetyltransferase to regulate lysine 16 acetylation on histone 4 (H4K16Ac), thus contributing to hypoxia-regulated gene expression. Clinically, RP11-367G18.1 variant 2 was upregulated in ccRCC tissues, particularly metastatic ccRCC tissues, and it is linked to poor overall survival.
Conclusion
These findings demonstrate the prognostic value and EMT-promoting role of RP11-367G18.1 and indicate that this lncRNA may provide a therapeutic target for ccRCC.
1 INTRODUCTION
Renal cell carcinoma (RCC) is a common malignancy in adults and accounts for greater than 90% of all kidney cancers. RCC comprises heterogeneous malignancies arising from the kidney parenchyma. Multiple histological variants of RCC have been identified, and the clinical, genetic, and pathological features may exhibit diverse expression.1 Clear cell RCC (ccRCC) is the most common and aggressive RCC subtype. Approximately one-third of patients develop metastatic RCC that is associated with a high mortality rate.2 The prognosis for non-metastatic RCC is good, and this disease is considered curable.3 As RCC is resistant to radiotherapy and chemotherapy, nephrectomy remains an effective treatment for RCC.4 A number of signaling pathways such as those involving von Hippel–Lindau (VHL)/hypoxia-inducible factor (HIF) and PI3K/Akt/mTOR have been reported to be involved in the pathogenesis and progression of RCC. Recent studies have demonstrated the prevalence of mutations in epigenetic regulatory genes other than VHL in the contest of RCC.5, 6 Although multiple candidates for prognostic and predictive biomarkers are under investigation, there are currently no reliable biomarkers for RCC. Identifying the key factors responsible for promoting metastasis is an important unmet clinical need that serves both predictive and therapeutic purposes.
The hypoxic microenvironment is a common feature of most solid tumors and plays a critical role in the metastasis and recurrence of a number of cancers, including ccRCC.7 HIF transcription factors drive the transcription of hypoxia-responsive genes that are involved in epithelial–mesenchymal transition (EMT), angiogenesis, and glucose transport.8 HIF-1α expression has been linked to poor survival in ccRCC patients.9 The HIF-2α inhibitor exhibits anticancer activity in patients with VHL-associated ccRCC.10 Hence, understanding the underlying mechanism of hypoxia-induced ccRCC progression is crucial for cancer treatment.
Long non-coding RNAs (lncRNAs) are transcripts without protein-coding capacity that regulate numerous biological and cellular processes. Evidence indicates that lncRNAs act as regulators of epigenetic, transcriptional, and post-transcriptional networks.11 A number of lncRNAs have been reported to mediate hypoxia-regulated tumor progression and metastasis.12, 13 The aberrant expression of lncRNAs in tumors is associated with ccRCC progression.14 However, the molecular mechanisms of lncRNAs underlying the regulation of EMT and metastasis in ccRCC remain unclear. In this study, we demonstrated that the hypoxia-responsive lncRNA RP11-367G18.1 (ENSG00000230943, also known as LINC02541) functioned as an EMT regulator in ccRCC. RP11-367G18.1 variant 2 was upregulated in metastatic ccRCC and was associated with poor outcomes. Hypoxia induced tumor growth and metastasis through RP11-367G18.1 variant 2 upregulation, and RP11-367G18.1 variant 2 contributed to the epigenetic regulation of EMT-activating transcription factors.
2 MATERIALS AND METHODS
2.1 Patient samples
A total of 216 specimens (149 ccRCC tumor samples and 67 relatively normal kidney parenchyma tissue samples) were collected from 149 patients between January 2015 and December 2020. All tissue samples were collected immediately after the diseased kidneys were removed during radical nephrectomy and were stored in liquid nitrogen prior to RNA extraction. The clinical data from all patients were collected by reviewing their medical charts and radiographic images. Preoperative general characteristics, including sex, age, body height, body weight, body mass index, underlying disease, Eastern Cooperative Oncology Group performance status, and American Society of Anesthesiologists score, were recorded. Data regarding tumor-related parameters such as tumor stage, pathological Fuhrman grade, renal vein invasion (RVI), and distant metastasis status were also collected (Table S1). Overall survival was recorded as the endpoint. Patients were followed-up for survival status until the time of the final.
2.2 RNA sequencing (RNA-seq)
The homogenized sample was collapsed by MagNA Lyser Green Beads (03358941001, Roche) with frozen tissue in RLT. RNA was purified by RNeasy followed the protocol of manual factory. The quality was assessed using a TapeStation (Agilent). After library constructed by KAPA Hyperprep kit with Ribominus, data were generated by Illumina NextSeq platform with 150 bp paired-end.
2.3 TCGA database analysis
Gene expression data and the survival status of the 602 RCC patients were analyzed using GDCRNAtools.15 Gene expression was Log2TMM-normalized using edgeR. Hazard ratios with log-rank p-value <0.05 were considered significant. The transcript levels of RP11-367G18.1 in patients with kidney renal clear cell carcinoma and survival status of patients were downloaded and analyzed using DriverDBv3 database.16
2.4 Cell culture
The RCC cells (Caki-1 and 786-O) were acquired from the American Type Culture Collection (Manassas, VA, USA) and cultivated in Dulbecco's Modified Eagle's Medium and RPMI 1640 Medium. UOK171 and A-498 cells were cultured in Dulbecco's Modified Eagle's Medium and Eagle's Minimum Essential Medium, respectively. All RCC cell lines were maintained in complete medium containing 10% fetal bovine serum at 37°C in a 5% CO2 incubator. For induction of hypoxia, RCC cells were cultured in 94% N2, 5% CO2, and 1% O2 for 18 h.
2.5 Plasmid construction, siRNA transfection, transduction, and reporter assay
The expression constructs pHA-HIF-1α, pHA-HIF-1α (ΔODD), and pHA-HIF-1α (LCLL) containing cDNA encoding wild-type, constitutively active, and inactive HIF-1α were kindly provided by L. Eric Huang (University of Utah).17 Plasmids expressing lncRNA RP11-367G18.1 variant 1 (ENST00000427157.1) and RP11-367G18.1 variant 2 (ENST00000452675.1) were constructed into pcDNA3.1 (+) vector as previously described.18 For gene knockdown, the target sequences of HIF-1α (5′-GTGATGAAAGAATTACCGAAT-3′) and RP11-367G18.1 variant 2 (5′-GGTTCTACTTCCTGGCAAGTA-3′) were cloned into a pLV2-U6-Puro lentiviral vector.18 For transfection, RCC cells were transfected using Lipofectamine 2000 (Thermo Fisher Scientific) following the manufacturer's instructions. For the lentiviral siRNA knockdown experiment, shRNA targeting RP11-367G18.1 variant 2 was inserted into a pLV2-U6-Puro vector for lentivirus production.18 RCC cells were transduced with the lentivirus and selected using puromycin for 2 weeks. Reporter plasmids containing RP11-367G18.1 promoter with wild-type or mutant hypoxia response element (HRE) were co-transfected with HIF-1α, LCLL, or control plasmids into Caki-1 cells for 48 h. The Renilla luciferase was used as an internal control. The luciferase activity was determined using the dual-luciferase reporter assay system (Promega).
2.6 Quantitative real-time PCR
Briefly, total RNA in both cells and tissues was prepared using TRIzol reagent, and the cytoplasmic and nuclear RNAs were purified using the SurePrep Nuclear or Cytoplasmic RNA Purification Kit (Thermo Fisher Scientific) for cDNA synthesis, as previously described.19 Quantitative real-time PCR was performed with primers (Table S2) using Fast SYBR Green Master Mix (Thermo Fisher Scientific). The relative transcript levels were normalized to 18 S.
2.7 RNA pull-down and western blot analysis
For RNA pull-down, the procedure was performed with minor modifications.20 Briefly, biotin-labeled RP11-367G18.1 variant 2 was synthesis by in vitro transcription and then purified. The biotinylated RP11-367G18.1 variant 2 was incubated with cell lysates at 4°C for 6 h. The streptavidin agarose beads were added into the mixture and incubated for 1 h. The beads were washed and boiled for western blot analysis. As previously described,18 proteins and histones extracted from the cells were analyzed by SDS–PAGE using antibodies against HIF-1α, N-cadherin (BD Biosciences), H4K5Ac, H4K8Ac, H4K12Ac, H4K20me3, H3K56Ac, histone H4, p300, Tip60, HBO1, HAT1, CBP (Abcam), E-cadherin, histone H3 (Cell Signaling Technology), plakoglobin, H3K4me2, H3K9Ac, H4K16Ac (Millipore), vimentin (Sigma-Aldrich), and β-actin (Genetex).
2.8 Migration, invasion, and soft agar colony formation assays
Cells were seeded onto cell culture inserts in 24-well plates for evaluation of migration (3 × 104 cells) and invasion (5 × 104 cells), respectively. Migrated or invaded cells were counted under a light microscope. For colony formation, RCC cells (5 × 103) suspended in 0.3% agarose gel were seeded onto 0.5% agarose gel. After 14 days of incubation, visible colonies were stained with crystal violet and counted under a light microscope.21
2.9 Xenograft tumorigenicity assay
Animal procedures were approved by the Institutional Animal Care and Use Committee of China Medical University. Briefly, BALB/c nu/nu mice (5-week-old) used as the in vivo tumor growth model were obtained from the National Science Council Animal Center (Taipei, Taiwan). Each mouse was inoculated with Caki-1 cells (2 × 106) subcutaneously. After inoculation at 30–35 days, the mice were sacrificed, and the tumor volume was measured. Tumor volume was calculated using a formula: π/6 × length × width × height.22
2.10 Tail vein and orthotopic metastatic mouse models
Male NOD-SCID mice (6-week-old, National Science Council Animal Center) were used to assess metastatic ability. For tail vein metastatic mouse models, Caki-1 cells were intravenously injected (1 × 106 cells) into the tail veins of NOD-SCID mice.23 For orthotopic metastatic mouse models, mice were anesthetized with isoflurane. The subcutaneous and muscle tissues were dissected, and the kidney was lifted upon the body surface from the incision. Caki-1 cells (1 × 105 cells) were orthotopically inoculated into renal capsule of NOD-SCID mice.24 After 15 weeks, the mice were sacrificed and the lung of mice was surgically removed. The pulmonary metastatic nodules in mice were observed and counted using a dissecting microscope.
2.11 Chromatin immunoprecipitation (ChIP) and chromatin isolation by RNA purification (ChIRP) assays
As previously described,18, 25 the ChIP assay was performed using antibodies against IgG, HIF-1α (Diagenode), H4K16Ac, and p300 (Abcam). The immunoprecipitated DNAs were amplified using specific primers (Table S2) and quantified by real-time PCR. The percentage of immunoprecipitated DNAs in the input DNA was calculated. The ChIRP assay was performed using RP11-367G18.1 variant 2 and lacZ probes (generated from online design software: singlemoleculefish.com; Table S3) as previously described.26
2.12 Single-molecule RNA fluorescent in situ hybridization (RNA-FISH) and immunofluorescence staining
The RNA-FISH probes targeting RP11-367G18.1 variant 2 were designed using a Stellaris Probe Designer and were purchased from LGC Biosearch Technologies (Table S4). Briefly, cells or tissues were fixed with 4% formaldehyde for 15 min at room temperature, washed, and permeabilized as previously described.18 Samples were incubated in a hybridization solution with RNA-FISH probes. Hybridization was performed for 4 h at 55°C avoiding light. Samples were washed for three times, blocked with 1% BSA for 1 h, and subsequently incubated with anti-HIF-1α (1:100 dilution, abcam) or anti-H4K16Ac (1:250 dilution, abcam) antibody at 4°C overnight. Then, samples were washed and incubated with secondary antibody solution (1:500 dilution; goat anti-rabbit Alexa488, abcam) containing a 1:10000 dilution of the DAPI stock solution for 1 h at room temperature. Images were acquired using a Leica TCS SP8 STED microscope and analyzed using MetaMorph software version 7.8.0.0 (Universal Imaging) and Leica Application Suite X (LAS X version 3.5.5.19976) software. RP11-367G18.1 variant 2 in cells were counted from three independent replicates.
2.13 Statistical analysis
Statistical significance was defined as a p-value <0.05. Statistical comparisons were performed using the Student's t-test. The overall survival curve of patients with ccRCC was depicted using the Kaplan–Meier method. To compare the survival distributions, the log-rank test was used. Univariate and multivariate logistic regression models were applied to identify pathophysiological characteristics that associated with metastasis in patients with ccRCC.
3 RESULTS
3.1 RP11-367G18.1 is upregulated in ccRCC tissues and linked to worse outcome
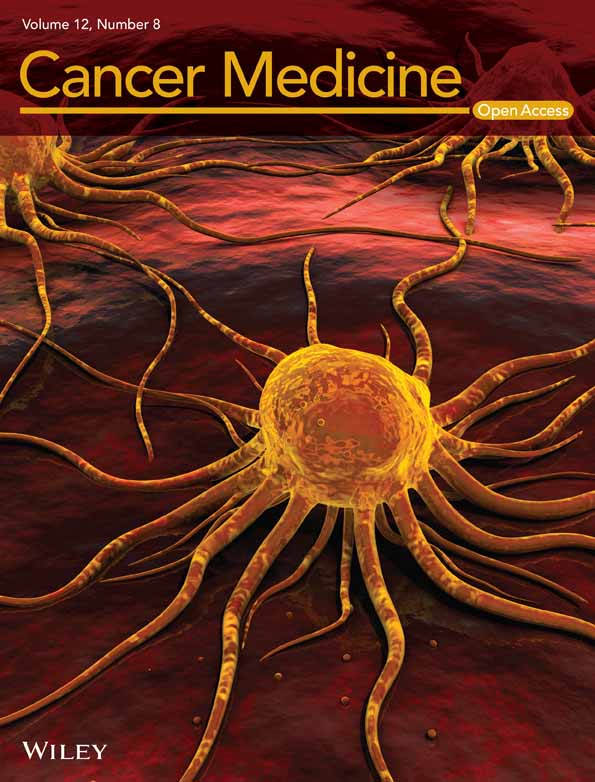
To examine the important tumor progression factors, we performed RNA-seq and ATAC-seq on clinical samples of ccRCC (two normal kidney parenchyma samples and three tumor samples). Using RNA-seq analysis, we identified 536 upregulated genes in tumor versus normal tissues with a cut-off 1.5-fold change and a p-value <0.05 (Figure 1A). To further investigate the epigenetic regulation mechanism of 536 significantly upregulated genes in tumors, we applied differential chromatin accessibility analysis and focused on the accessibility change at the promoter after annotation of the peak sets (Figure S1A). There was a positive correlation between chromatin accessibility and gene expression (Figure S1B). The results of the comparison of chromatin accessibility and significantly upregulated genes indicated that most of the accessible regions were increased in tumor samples (201 increased chromatin accessibility promoter regions, 86 genes). We focused on the upregulated genes with increased chromatin accessibility promoter regions (Figure 1B). The gene ontology analysis revealed that the HIF-1 signaling was the most remarkably enriched KEGG pathway (Figure 1C). These data suggested that HIF-1 might be an important factor for increasing chromatin accessibility in tumors, and therefore, we compared the promoter region of increased accessibility to previous HIF-1α ChIP-seq data sets (Table S5). Based on this, we identified 19 genes containing HIF-1 binding sites with higher chromatin accessibility in the tumor (Figure 1D). Several well-known hypoxia-induced oncogenic proteins in RCC have been identified, including CAV1, CA9, and VEGFA.27-29 We focused on lncRNAs to explore the novel mechanism of noncoding epigenetic regulation and performed survival analysis using TCGA data. The data revealed that lncRNA PVT1 and RP11-367G18.1 exhibited higher hazard ratios in RCC patients (Figure 1E,F).
3.2 Hypoxia-induced RP11-367G18.1 enhances EMT
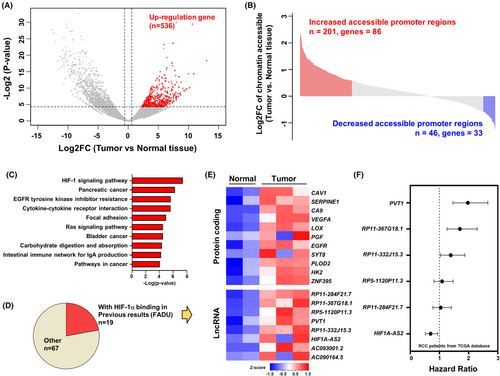
Based on GRCh37 assembly in Ensembl, there were two transcripts of RP11-367G18.1 (variants 1 and 2; Figure S2A). CcRCC cell lines harbored higher levels of endogenous HIF-1α had higher RP11-367G18.1 variant 2 expressions (786-O and A-498 cells), while cell with lower levels of endogenous HIF-1α had lower RP11-367G18.1 variant 2 expressions (Caki-1 and UOK171 cells; Figure S2B). To validate if RP11-367G18.1 is modulated by hypoxia/HIF-1α, the transcript levels of RP11-367G18.1 were measured. The levels of RP11-367G18.1 transcripts were increased under hypoxic conditions in Caki-1 and UOK171 cells (Figures 2A and S2C,D). Conversely, the levels of RP11-367G18.1 transcripts were decreased by HIF-1α knockdown in 786-O and A-498 cells (Figures 2B and S2E,F). Hypoxia-induced RP11-367G18.1 expression was diminished following HIF-1α knockdown (Figure 2C). Both wild-type HIF-1α and constitutively active HIF-1α (HIF-1α (ΔODD)) increased RP11-367G18.1 expression (Figure 2D). Accordingly, we performed the reporter assay and found that the activity of RP11-367G18.1 promoter with wild-type HRE responded to hypoxia and HIF-1α overexpression but not inactive HIF-1α (LCLL). However, hypoxia/HIF-1α-induced activity of RP11-367G18.1 promoter was abolished following HRE mutation (Figure S2G). Furthermore, the results of the ChIP assay suggested that HIF-1α bound to RP11-367G18.1 promoter with HRE (Figure S2H). To determine which variant was a key regulator of EMT, we constructed expression vectors from the two variants of RP11-367G18.1. The results demonstrated that variant 2 of RP11-367G18.1 inhibited the levels of E-cadherin and plakoglobin (epithelial markers) and enhanced the levels of vimentin and N-cadherin (mesenchymal markers) that was accompanied by the induction of cell migration and invasion, while variant 1 did not affect these processes (Figures 2E,F and S2I–K). In contrast, knockdown of RP11-367G18.1 variant 2 suppressed cell migration and invasion (Figures 2G and S2L). These results confirmed that RP11-367G18.1 was directly regulated by HIF-α and contributed to EMT.
3.3 RP11-367G18.1 variant 2 mediates hypoxia-induced EMT
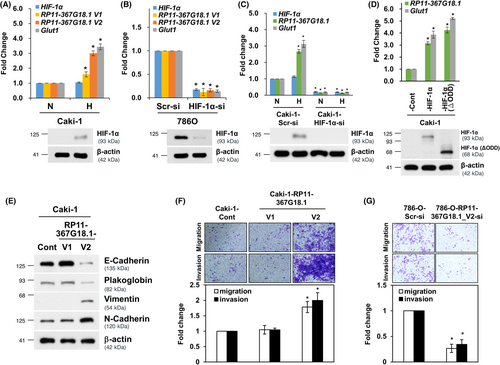
We then explored the role of RP11-367G18.1 variant 2 in hypoxia/HIF-1α elicited EMT, and we observed that inhibition of RP11-367G18.1 variant 2 reversed the hypoxia-induced reduction in epithelial markers and the induction of mesenchymal markers (Figures 3A and S3A). The increased cell migration and invasion under hypoxia were suppressed by RP11-367G18.1 variant 2 knockdown (Figure 3B). Similarly, overexpression of constitutively active HIF-1α induced EMT phenotypes, and this was inhibited by knockdown of RP11-367G18.1 variant 2 (Figures 3C,D and S3B). These results suggested that hypoxia/HIF-1α signaling induced EMT through RP11-367G18.1 variant 2.
3.4 HIF-1α enhances the tumorigenicity and metastasis of ccRCC through RP11-367G18.1 variant 2
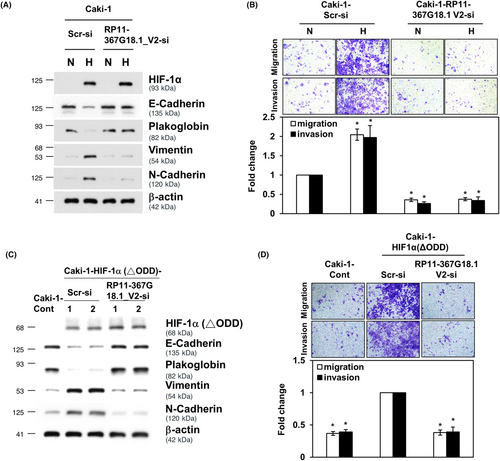
To evaluate the effect of RP11-367G18.1 variant 2 on the tumorigenicity of ccRCC, soft agar colony formation assay and tumor xenograft experiments were performed. The data revealed that ectopic expression of RP11-367G18.1 variant 2 promoted colony formation and tumor growth of RCC (Figures 4A,B and S4A). Furthermore, ectopic expression of HIF-1α (ΔODD) promoted colony formation in RCC cells, and this was repressed by knockdown of RP11-367G18.1 variant 2 (Figure 4C). Consistently, knockdown of RP11-367G18.1 variant 2 significantly reduced the tumor growth of xenografted RCC cells overexpressing constitutively active HIF-1α (Figure 4D). To evaluate the effect of RP11-367G18.1 variant 2 on metastasis, NOD-SCID mice were used for the tail vein and orthotopic metastatic mouse models. The data revealed that the number of metastatic lung nodules was raised following overexpression of RP11-367G18.1 variant 2 (Figure 4E and S4B). Knockdown of RP11-367G18.1 variant 2 reduced metastatic ability caused by overexpressing constitutively active HIF-1α in vivo (Figure 4F). The above data suggested that hypoxia-driven ccRCC metastasis occurred through the upregulation of RP11-367G18.1 variant 2.
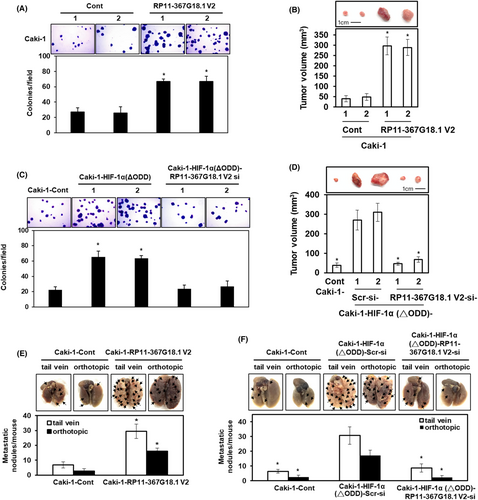
3.5 RP11-367G18.1 variant 2 increases lysine 16 acetylation on histone 4 and facilitates hypoxia-regulated gene expressions
The subcellular localization of lncRNAs is linked to its function.30 We observed that nuclear RP11-367G18.1 variant 2 was highly upregulated under hypoxia (Figure S5A). Then, we explored the distribution of RP11-367G18.1 variant 2 and found that RP11-367G18.1 variant 2 mainly localized in the nucleus (Figure S5B,C). Notably, tissue with high levels of RP11-367G18.1 variant 2 were associated with high levels of HIF-1α in the xenografts and the human ccRCC tissues (Figure S5D,E). The nuclear lncRNAs can function as epigenetic regulators by interacting with histone modifiers.31 To determine the effect of RP11-367G18.1 on histone modification, changes in histone marks were examined. The data revealed that the level of histone 4 lysine 16 acetylation (H4K16Ac) was decreased in RP11-367G18.1-knockdown cells (Figure 5A). Overexpression of RP11-367G18.1 variant 2 enhanced H4K16Ac levels, while variant 1 did not affect this modification (Figures 5B and S6A). Hypoxia-induced H4K16Ac activation was attenuated by RP11-367G18.1 variant 2 knockdown (Figure 5C). Moreover, RP11-367G18.1 variant 2 and H4K16Ac mark were colocalized in the nucleus (Figure S5B). The H4K16Ac modification has been reported to activate gene transcription.32 Therefore, we examined the expression of hypoxia-regulated target genes, and we observed that knockdown of RP11-367G18.1 variant 2 remarkably decreased the mRNA expressions of Twist1, SLUG, and VEGF under hypoxic conditions (Figure 5D). Hypoxia-induced Twist1 and SLUG protein expression was suppressed by knockdown of RP11-367G18.1 variant 2 (Figure 5E). Ectopic expression of RP11-367G18.1 variant 2 increased the protein levels of Twist1 and SLUG (Figure 5F). Conversely, inhibition of RP11-367G18.1 variant 2 suppressed the mRNA and protein expressions of Twist1 and SLUG (Figures 5G,H and S6B,C). To validate which histone modifier mediated RP11-367G18.1 variant 2-induced H4K16Ac, the association of RP11-367G18.1 variant 2 with common histone acetyltransferases was examined. The results of RNA pull-down assay suggested that p300 interacted with biotinylated RP11-367G18.1 variant 2 (Figure 5I). Importantly, the decreased levels of H4K16Ac, p300, and RP11-367G18.1 variant 2 on the proximal promoters of Twist1, SLUG, and VEGF under hypoxia following RP11-367G18.1 variant 2 knockdown (Figure 5J–L). These results indicated that RP11-367G18.1 variant 2 interacted with p300 and induced the H4K16Ac marks to regulate hypoxia-induced target genes.
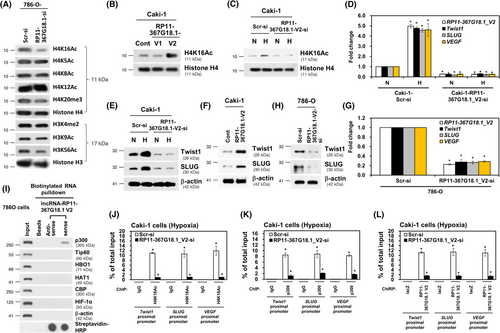
3.6 RP11-367G18.1 variant 2 is linked to metastasis and unfavorable survival
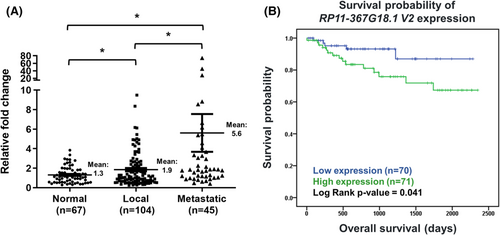
Finally, we explored the clinical significance of RP11-367G18.1, data from TCGA-KIRC (kidney renal clear cell carcinoma) cohort was downloaded and analyzed. Primary solid tumors possessed higher RP11-367G18.1 transcripts than normal tissues (Figure S7A). Patients with high expression of RP11-367G18.1 had worse overall survival, progression-free interval, and disease-specific survival (Figure S7B). Due to lack of lncRNA isoform data in the TCGA database, we collected specimens from patients with ccRCC and performed real-time PCR analysis using RP11-367G18.1 variant 2-specific primer. Our in-house data showed that upregulation of RP11-367G18.1 variant 2 in ccRCC tumors compared to levels in normal parenchyma. Moreover, metastatic ccRCC tissues harbored higher levels of RP11-367G18.1 variant 2 than localized ccRCC tissues (Figure 6A). High RP11-367G18.1 variant 2 level exhibited poor overall survival of ccRCC patients (Figure 6B). Univariate and multivariate logistic analyses revealed that tumor stage and RP11-367G18.1 variant 2 expressions were independent prognostic indicators for distant metastasis (Table S6). These findings suggested the oncogenic and EMT-promoting roles of RP11-367G18.1 variant 2 in ccRCC.
4 DISCUSSION
CcRCC tends to be more advanced stage at diagnosis and is associated with high mortality. Genetic defect of VHL, the negative regulator of HIFs, is a frequent genetic alteration of ccRCC that leads to increased HIF target gene expression.4, 33 A meta-analysis indicates that increased nuclear expression of HIF-1α and cytoplasmic expression of HIF-2α are linked to unfavorable prognosis in patients with RCC.34 HIF-1α and HIF-2α have been reported to play different roles in ccRCC tumor development and inflammation.35 Belzutifan, a selective small-molecule inhibitor of HIF-2α, is recently approved by the U.S. Food and Drug Administration for the systemic treatment of VHL–associated RCC.36 Therapeutic strategies for RCC treatment that target HIF-1α and its downstream target genes are worthy of further exploration.
The ability of cells to migrate and invade is a hallmark of EMT and is associated with metastasis. EMT-activating transcription factors, including Twist1, Snail, and ZEB family members, play a pivotal role in cancer progression.37 To identify prognostic biomarkers for ccRCC, hypoxia-related and EMT-related lncRNAs have been previously explored.33, 38 The emerging role of hypoxia-responsive lncRNAs in tumor growth and metastasis has become a popular research focus.39 For example, lncRNA PVT1 is upregulated by HIF-2α and then interacts with HIF-2α and prevents its degradation. PVT1 enhances tumor progression and metastasis and is associated with worse ccRCC outcomes.40 Interestingly, we also identified PVT1 as an HIF-1-related lncRNA that was upregulated in ccRCC tissues and was linked to decreased survival (Figure 1E,F). Recently, the EMT-promoting role of hypoxia-induced RP11-367G18.1 has been reported. High levels of RP11-367G18.1 are linked to shorter survival in patients with head and neck cancer.18
LncRNAs can scaffold and recruit multiple regulatory factors to integrate functions, including histone modifications. LncRNAs can directly interact with writers, readers, and erasers of histones.41 The lncRNA HOTAIR interacts with the polycomb complex PRC2 and promotes gene repression through H3K27 methylation.42 LncRNA IL-7–AS binds p300 to facilitate histone acetylation, thus promoting inflammatory gene transcription.43 High levels of H3 and H4 acetylation are associated with the promoters of active genes.32 Our findings revealed that hypoxia upregulated lncRNA RP11-367G18.1 variant 2 which activated H4K16Ac marks in ccRCC. We identified p300, a H4K16Ac acetyltransferase,44 that interacted with RP11-367G18.1 variant 2. RP11-367G18.1 variant 2-p300 complex activated H4K16Ac marks on the promoters of hypoxia-regulated gene to induce transcription. These hypoxia-regulated genes, such as Twist, SLUG, and VEGF, promoted ccRCC progression and metastasis (Figure 7).
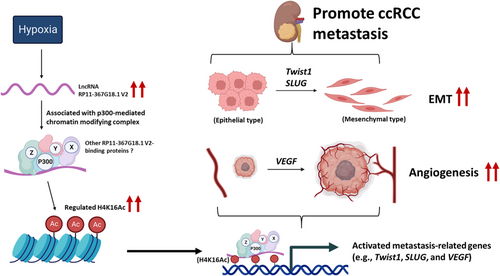
Our results revealed that an increased level of RP11-367G18.1 variant 2 in metastatic ccRCC tissues was linked to decreased survival. RP11-367G18.1 variant 2 was upregulated by hypoxia and HIF-1α that mediated HIF-1α-induced tumor growth and metastasis. RP11-367G18.1 variant 2 elevated H4K16Ac levels to thereby modulate hypoxia-regulated gene expression. In conclusion, hypoxia-induced RP11-367G18.1 variant 2 serves as a novel EMT activator, thus suggesting its potential as a prognostic biomarker and molecular target in ccRCC.
AUTHOR CONTRIBUTIONS
I-Hung Shao: Data curation (equal); investigation (equal); methodology (equal); validation (equal); writing – original draft (equal). Pei-Hua Peng: Data curation (equal); investigation (equal); methodology (equal); validation (equal). Heng-Hsiung Wu: Data curation (equal); investigation (equal); methodology (equal); validation (equal). Ji-Lin Chen: Investigation (equal); resources (equal); writing – original draft (equal); writing – review and editing (equal). Joseph Chieh-Yu Lai: Data curation (equal); methodology (equal); validation (equal); visualization (equal). Jeng-shou Chang: Methodology (equal); resources (equal); validation (equal). Han-Tsang Wu: Data curation (equal); methodology (equal); resources (equal). Kou-Juey Wu: Conceptualization (equal); methodology (equal); resources (equal). See-Tong Pang: Funding acquisition (equal); resources (equal); supervision (equal). Kai-Wen Hsu: Conceptualization (equal); funding acquisition (equal); supervision (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
UOK171 and A-498 cells were kindly provided by Dr. Cheng-Keng Chuang (Chang Gung Memorial Hospital at Linkou).
FUNDING INFORMATION
This study was supported by grants from the Ministry of Science and Technology Summit and Frontier grants (MOST 109-2320-B-182A-022, MOST 109-2628-B-039-006, MOST 110-2326-B-182A-004, MOST 110-2320-B-182A-019, MOST 110-2628-B-039-007, MOST 110-2314-B-371-007-MY3, MOST 111-2628-B-039-007-MY3, MOST 111-2314-B-182A-034), Chang Gung Memorial Hospital (OMRPG3I0012, NMRPG3J6192, CORPG3J0232, NMRPG3J0672, NMRPG3K0511, NRRPG3L0151, NRRPG3M0081, CORPG3J0241–3, CMRPG3L0981), China Medical University (CMU110-MF-10, CMU111-MF-05), and the “Drug Development Center, China Medical University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project (Ministry of Education, Taiwan).
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: Chang Gung Memorial Hospital (IRB: 202001634B0). Informed Consent: All informed consent was obtained from the subject(s). Registry and the Registration No. of the study/trial: N/A. Animal Studies: China Medical University (IACUC: CMUIACUC-2019-022-4).
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.