Psychosocial factors impacting barriers and motivators to cancer genetic testing
Abstract
Background
Only a small proportion of patients who qualify for clinical genetic testing for cancer susceptibility get testing. Many patient-level barriers contribute to low uptake. In this study, we examined self-reported patient barriers and motivators for cancer genetic testing.
Methods
A survey comprised of both new and existing measures related to barriers and motivators to genetic testing was emailed to patients with a diagnosis of cancer at a large academic medical center. Patients who self-reported receiving a genetic test were included in these analyses (n = 376). Responses about emotions following testing as well as barriers and motivators prior to getting testing were examined. Group differences in barriers and motivators by patient demographic characteristics were examined.
Results
Being assigned female at birth was associated with increased emotional, insurance, and family concerns as well as increased health benefits compared to patients assigned male at birth. Younger respondents had significantly higher emotional and family concerns compared to older respondents. Recently diagnosed respondents expressed fewer concerns about insurance implications and emotional concerns. Those with a BRCA-related cancer had higher scores on social and interpersonal concerns scale than those with other cancers. Participants with higher depression scores indicated increased emotional, social and interpersonal, and family concerns.
Conclusions
Self-reported depression emerged as the most consistent factor influencing report of barriers to genetic testing. By incorporating mental health resources into clinical practice, oncologists may better identify those patients who might need more assistance following through with a referral for genetic testing and the response afterwards.
1 INTRODUCTION
An estimated 5%–10% of all cancers have a hereditary cause yet many patients who meet National Comprehensive Cancer Network (NCCN) guidelines for clinical genetic testing for cancer susceptibility do not undergo testing. Guidelines suggest testing is indicated for all patients with pancreatic adenocarcinoma, however, only around 20% of patients get tested.1 Patients with prostate cancer have a low rate of germline genetic testing uptake with one study finding that only 11% of the eligible patients completed testing.2 Fewer than 20% of breast and ovarian cancer patients who qualify for testing based on NCCN guidelines receive testing.3, 4 Another study found that among women with breast cancer who qualify for genetic testing per NCCN guidelines, only about 60% were referred and about 46% completed testing indicating that despite a referral, there is still a reduction of uptake in germline testing.5 Patients at risk for Lynch Syndrome diagnosed with either colorectal or endometrial cancer have even lower levels of testing with one study reporting a rate around 6% and another study reporting a rate around 3%.6, 7 Germline genetic testing for patients with cancer can have significant impact on their cancer treatment and surveillance as well as implications for family members.
Reasons for low uptake of genetic testing can be broken down into patient, provider, and systemic barriers. Patient-level factors, defined as factors impacting or perceived by an individual patient, include lack of provider referral and concerns about cost, clinical utility, privacy and confidentiality, and logistical issues.8-11 The provider reported barriers to testing include perceived patient disinterest, long wait times for genetic services, insurance coverage concerns, unknown guidelines for management, insufficient experience/training, and clinical time limitations.12-15 Systemic barriers include reduced access to specialists for under- and uninsured patients, health system mistrust, and lack of pre-symptomatic genetic testing with public insurance.16-18 Although there is suboptimal utilization of genetic testing across all cancer types, much of the current literature regarding the barriers to genetic testing focuses on patients with breast and ovarian cancer. Less is known about barriers among patients with other cancer types. Additionally, there is a gap in the literature investigating patient-level motivators for genetic testing.
In this study, we aimed to identify patient-level barriers and motivators to genetic testing across multiple cancer types. Within the barrier and motivator domains, we created empirically derived subscales for emotional concerns, insurance concerns, social and interpersonal concerns, family worry, internally motivated emotions, health benefits, and interpersonal emotions. We hypothesized that sex, age, having children, and diagnosis of a cancer type associated with hereditary cancer syndromes would be associated with an increase in specific barriers to testing. Specifically, we hypothesized that (1) those assigned female at birth would have increased family concerns and social and interpersonal concerns based on prior literature19-21; (2) younger respondents would have increased scores on all barrier subscales and the increased health benefits motivator subscale as cancer is often regarded as a disease related to older age21-23; (3) having children would be associated with increased scores on the family concern subscale; (4) having a diagnosis of a BRCA-associated cancer type would be associated with higher scores on the social and interpersonal concerns subscale and the health benefits subscale due to the prevalence of information regarding BRCA 1/2 testing implications.24
2 METHODS
2.1 Study design and cohort selection
This cross-sectional study was conducted to better understand barriers and drivers for genetic testing and ultimately inform an intervention being deployed a as part of a larger randomized trial. We queried the University of Michigan patient registry to identify individuals with a documented in-patient or out-patient encounter at Michigan Medicine between January 1, 2019, and February 15, 2021, with an ICD-9/10 code for cancer with one of the following characteristics: diagnosed under the age of 50 for breast or prostate cancer, or any age for colorectal, endometrial, ovarian, pancreatic, or metastatic prostate cancer. These eligibility criteria were selected for patients who likely qualified for genetic testing by NCCN guidelines. Participants were shown a list of 21 cancer types (bladder, bone, breast, cervical, colon, endometrial, head and neck, Hodgkin's lymphoma, leukemia, liver, lung, melanoma, non-Hodgkin's lymphoma, oral, ovarian, pancreatic, prostate, rectal, renal, non-melanoma skin, and stomach) and selected all primary cancer types that they had been diagnosed with in the past. Medical chart review was not performed to confirm diagnoses. Of the 376 respondents, 8 (2.1%) did not report one of the six original cancers but were included in the analysis as they did report a diagnosis of cancer, received genetic testing despite a lack of NCCN guidelines, and we felt that their psychosocial barriers and motivators would align with the rest of the group.
2.2 Ethical considerations
The University of Michigan Medical School Institutional Review Board (IRB) approved this study, which involved contacting patients who met the eligibility criteria via email (HUM0019157). Potential participants were presented with a consent and waiver of formal written consent was granted by the IRB.
2.3 Measures
Demographic variables collected include sex assigned at birth, age, race/ethnicity, education, income level, insurance status, number of biological children, and employment status. Patients were asked to report all primary cancer types, date of cancer diagnosis, treatment received, and genetic testing status. The PHQ-4 was used to measure anxiety and depression; respondents scores were dichotomized by either none (0–2) or mild to severe (3–12).25 As part of the survey, participants were asked if they had undergone cancer genetic testing. For this analysis, only participants who had reported receiving germline cancer genetic testing were included.
We gathered individual questions from existing measures of knowledge, attitudes, and perceptions of barriers and benefits of genetic testing, adding new items where needed.11, 14, 26-31 Barrier items in the survey encompassed several domains including worry, fear, limited genetics knowledge, cost, impact of testing, psychosocial distress, genetic discrimination, stigma, religious beliefs, and logistics such as healthcare utilization. Motivators included emotional impact and actionable knowledge leading to changes in healthcare. As testing was already completed, participants were asked to recall how true 33 barriers and nine motivators were to them before they had genetic testing using a 5-point Likert scale (1 = not true at all, 5 = very true). Emotional responses after genetic testing were assessed using the validated, 12-item Feelings About genomic Testing Results (FACToR) questionnaire (per subscale Cronbach's α 0.66, 0.78, 0.72, 0.70) which asks respondents to recall how they felt in the past week using a 6-point Likert scale (1 = Not at all, 6 = A great deal).32
2.4 Study administration
An invitation to the study was sent by email with link to a Qualtrics survey was sent to 3000 potentially eligible individuals who were offered $10 upon completion. No reminders were sent. We targeted a sample of 350 respondents who had completed genetic testing to allow for subgroup comparisons.
2.5 Statistical analyses
Each domain- barrier, motivator, and FACToR- was analyzed separately to ensure the largest possible sample size for each analysis. For each set of measures (barriers, motivators, and FACToR), half of the data (split randomly) was used for an exploratory factor analysis (EFA), with the other half used for a confirmatory factor analysis (CFA). In EFA, parallel analysis was used to determine the number of latent factors present in the data, and factors were computed using principal axis factoring with a promax rotation to allow for correlated factors while maintaining a simple structure. The fit of the models identified in EFA were assessed in CFA models on the other half of the data. CFA models were fit using diagonally weighted least squares estimation with robust standard errors. CFA model fit was evaluated by considering comparative fit index (CFI), Tucker–Lewis index (TLI), root mean square error approximation (RMSEA), and the chi-squared test statistic. Multiple indicators multiple causes (MIMIC) models were used to assess the effects of demographics and patient characteristics on the latent factors. Effects were considered statistically significant at the 0.05 level after controlling for multiple-testing with the Benjamini–Hochberg correction. The “lavaan” and “psych” packages in R were used to analyze the data.
For this analysis, BRCA-related cancer types included breast, ovarian, prostate, pancreatic, and melanoma, and Lynch-related cancer types included colorectal, endometrial, stomach, ovarian, pancreatic, bladder, liver, and kidney. Respondents could fall into both BRCA- and Lynch-related cancer type categories based on their cancer history. These cancer cluster syndromes were chosen because they are widely recommended for testing by clinicians and are most familiar to patients. Lynch was also chosen as it is not confounded by sex, as both males and females were found in the Lynch syndrome group.
3 RESULTS
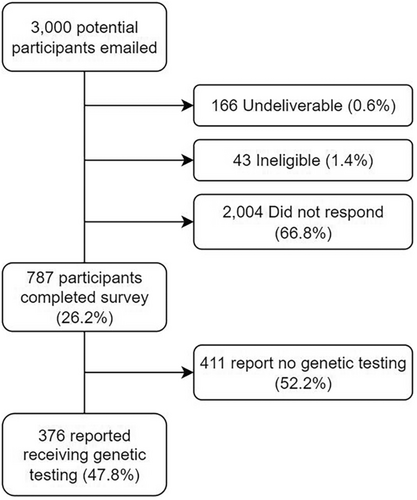
Of the 3000 potential participants invited via email 166 (0.6%) were undeliverable, 43 (1.4%) were ineligible mostly due to not having a diagnosis of cancer, and 2004 (66.8%) did not respond (Figure 1). Out of the 787 participants who responded, 47.8% (n = 376) reported having had genetic testing and were included in our analysis. Most of these tested participants were assigned female at birth (n = 347/376, 92.3%). Age ranged from 21 to 85 years old (median age of 48 years). Years since cancer diagnosis was distributed across ≤2 (n = 94/376, 25.0%), 2–5 (n = 152/376, 40.4%), 5–10 (n = 88/376, 23.4%), and > 10 (n = 42/376, 11.2%). The most common cancer diagnosis was breast (n = 235) followed by ovarian (n = 79), pancreatic (n = 25), colorectal (n = 25), endometrial (n = 15), and prostate (n = 10). A majority of respondents reported having biological children (n = 289/376, 76.9%). Only 6.1% (n = 22/376) respondents reported anxiety and 11.2% (n = 40/376) reported depression as assessed by the PHQ-4. Other demographics of the cohort are reported in Table 1.
| N | % | |
|---|---|---|
| Sex at birth | ||
| Female | 347 | 92.3 |
| Male | 29 | 7.7 |
| Race | ||
| American Indian or Alaska Native | 7 | 1.9 |
| Asian or Asian-American | 20 | 5.3 |
| Black or African American | 11 | 2.9 |
| Middle Eastern or North African | 9 | 2.4 |
| White, non-Hispanic | 338 | 90.1 |
| Missing | 1 | 0.3 |
| Age | ||
| ≤ 45 | 139 | 37.1 |
| 46–65 | 184 | 49.1 |
| ≥ 66 | 52 | 13.9 |
| Missing | 1 | 0.3 |
| Education | ||
| Less than bachelors | 96 | 25.5 |
| Bachelors | 131 | 34.8 |
| Advanced degree | 149 | 39.6 |
| Income | ||
| < $30,000 | 24 | 7.3 |
| $30,000–$74,999 | 88 | 26.7 |
| ≥ $75,000 | 218 | 66.1 |
| Missing | 46 | 12.2 |
| Health insurance | ||
| Public/government | 79 | 21.0 |
| Private | 291 | 77.4 |
| Other sources | 4 | 1.1 |
| Missing | 2 | 0.5 |
| Years since cancer diagnosis | ||
| ≤ 2 | 94 | 25.0 |
| 2–5 | 152 | 40.4 |
| 5–10 | 88 | 23.4 |
| > 10 | 42 | 11.2 |
| BRCA 1 / 2-related cancer | ||
| No | 41 | 10.9 |
| Yes | 335 | 89.1 |
| Lynch syndrome-related cancer | ||
| No | 266 | 70.3 |
| Yes | 110 | 29.3 |
| Have children | ||
| No | 87 | 23.1 |
| Yes | 289 | 76.9 |
| Anxiety | ||
| No | 336 | 93.9 |
| Yes | 22 | 6.1 |
| Missing | 18 | 4.8 |
| Depression | ||
| No | 318 | 88.8 |
| Yes | 40 | 11.2 |
| Missing | 18 | 4.8 |
Results of the CFA for the three a priori domains – barriers, motivators, and FACToR questions can be found in Table 2. The barrier domain items best fit with a four-factor solution (CFI = 0.93, TLI = 0.92) which we labeled emotional concerns (four items), insurance concerns (three items), social and interpersonal concerns (six items), and family concerns (three items). Emotional concerns include feeling scared, angry, hopeless, and anxious. Insurance concerns related to both life and health insurance. Social and interpersonal concerns related to feeling different or “other” when compared to the people around them. Family concerns, the fourth scale, assessed concerns over potential for family members developing cancer or finding pathogenic variants.
| Retrospective barriers domains | ||
|---|---|---|
| CFI = 0.93, TLI = 0.92, RMSEA = 0.061, χ2 = 164.11, p < 0.001 | ||
| Factor 1 | Emotional concerns | |
| I thought if I were found to carry an altered gene, I worried I would feel … scared | ||
| I thought if I were found to carry an altered gene, I worried I would feel … angry | ||
| I thought if I were found to carry an altered gene, I worried I would feel … hopeless | ||
| I thought if I were found to carry an altered gene, I worried I would feel … anxious | ||
| Factor 2 | Insurance concerns | |
| I worried about how it would affect my health insurance. | ||
| I worried it would be considered a pre-existing condition for health insurance if I were found to carry an altered gene. | ||
| I worried it would affect my life insurance policy if I were found to carry an altered gene. | ||
| Factor 3 | Social and interpersonal concerns | |
| I thought that if I carried an altered gene, it would cause me to feel less healthy than other people. | ||
| I thought that if I carried an altered gene, it would cause others to view me negatively. | ||
| I worried that if I carried an altered gene, I would feel singled out. | ||
| I was concerned about my partner's reaction to my genetic testing results. | ||
| I was concerned about my family's reaction to my genetic testing results. | ||
| I thought if I were found to carry an altered gene, I worried I would feel … ashamed | ||
| Factor 4 | Family concerns | |
| I thought that if I carried an altered gene, it would cause me to worry more about other family members who could be carriers. | ||
| I thought that if I carried an altered gene, I would feel guilty if my family member developed cancer. | ||
| I would feel guilty if one of my relatives had an altered gene and I did not. | ||
|
Retroactive motivator domains CFI = 0.94, TLI = 0.91, RMSEA = 0.065, χ2 = 42.36, p = 0.012 |
||
| Factor 1 | Internally motivating emotions | |
| I thought if I was found to carry an altered gene, I would feel … Prepared | ||
| I thought if I was found to carry an altered gene, I would feel … Knowledgeable | ||
| I thought if I was found to carry an altered gene, I would feel … Relieved to know | ||
| I thought if I was found to carry an altered gene, I would feel … Empowered | ||
| Factor 2 | Health benefits | |
| Knowing my genetic status could help me plan my treatment. | ||
| Knowing whether or not I carry an altered gene would increase my sense of personal control. | ||
| Knowing that I carry an altered gene would help me decide whether to go for more frequent cancer screening. | ||
| Factor 3 | Interpersonal emotions | |
| I thought if I was found to carry an altered gene, I would feel … Responsible | ||
| I thought if I was found to carry an altered gene, I would feel … Purposeful | ||
|
FACToR domains CFI = 0.78, TLI = 0.68, RMSEA = 0.096, χ2 = 98.88, p < 0.001 |
||
| Factor 1 | Negativity/uncertainty | |
| How upset did you feel about your genetic test result? | ||
| How anxious or nervous did you feel about your genetic test result? | ||
| How sad did you feel about your genetic test result? | ||
| How frustrated did you feel that there are no definite disease prevention guidelines for you? | ||
| How uncertain did you feel about your genetic test result? | ||
| How uncertain did you feel about what your genetic test result means for your child(ren) and/or family's risk of disease? | ||
| Factor 2 | Positive emotion | |
| How happy did you feel about your genetic test result? | ||
| How relieved did you feel about your genetic test result? | ||
| Factor 3 | Positive outlook | |
| How much did you feel that you understood clearly your choices for disease prevention or early detection? | ||
| How helpful was the information you received from your genetic test result in planning for the future? | ||
| Factor 4 | Privacy | |
| How concerned did you feel that your genetic test result would affect your health insurance status? | ||
| How concerned did you feel that your genetic test result would affect your employment status? | ||
For the motivator domain, the CFA yielded three factors (CFI = 0.94, TLI = 0.91) which we named internally motivating emotions (four items), health benefits (three items), and interpersonal emotions (two items). Internally motivating emotions assessed confidence-inducing and uplifting emotions such as feeling prepared, knowledgeable, relieved to know, and empowered. The health benefit items assessed the impact of knowing genetic status when making healthcare decisions. Interpersonal emotions, the third scale, assessed how testing may increase a sense of responsibility and purpose.
The third domain comprised items from the previously published FACToR scale. The published item loadings did not fit our data as well as the alternative model determined by the CFA which we chose to use instead in this analysis. The alternative model identified four factors (CFI = 0.78, TLI = 0.68) which we named negativity and uncertainty (six items), positive emotions (two items), positive outlook (two items), and privacy concerns (two items). Negativity and uncertainty include emotional responses such as being upset, anxious, sad, or frustrated as well as uncertainty about test results and the implications for family. Positive emotions include feeling happy or relieved about genetic test results. Positive outlook reflects feeling that genetic testing helped clarify future disease prevention and other healthcare planning. The fourth scale, privacy concerns, relates to worry that genetic testing results would affect health insurance or employment status.
3.1 Scale correlates
Barrier subscale scores (Table 3), Motivators subscale scores (Table 4), and FACToR subscale scores (Table 5) are shown for each group described below.
| Emotional concerns | Insurance | Social and interpersonal concerns | Family worry | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Est | p-Value | Est | p-Value | Est | p-Value | Est | p-Value |
| Sex (female vs. male) | 0.801 | 0.001 | 0.4 | 0.041 | 0.396 | 0.086 | 0.527 | 0.04 |
| Age | −0.337 | <0.001 | −0.1 | 0.126 | −0.192 | 0.019 | −0.211 | 0.04 |
| Children (yes vs. no) | 0.285 | 0.12 | −0.181 | 0.198 | 0.14 | 0.437 | 0.86 | <0.001 |
| Years since diagnosis | 0.103 | 0.048 | 0.157 | 0.014 | 0.046 | 0.447 | 0.068 | 0.261 |
| BRCA-associated cancer (yes vs. no) | 0.345 | 0.237 | 0.138 | 0.419 | 0.548 | 0.005 | 0.253 | 0.303 |
| Lynch-associated cancer (yes vs. no) | −0.06 | 0.73 | 0.018 | 0.893 | 0.176 | 0.303 | −0.048 | 0.811 |
| Anxiety (any vs. none) | −0.435 | 0.158 | −0.437 | 0.075 | −0.198 | 0.634 | −0.276 | 0.446 |
| Depression (any vs. none) | 0.938 | <0.001 | 0.375 | 0.07 | 1.018 | <0.001 | 0.738 | 0.005 |
| Internally motivated emotions | Health benefits | Interpersonal emotions | ||||
|---|---|---|---|---|---|---|
| Variable | Est | p-Value | Est | p-Value | Est | p-Value |
| Sex (female vs. male) | 0.188 | 0.668 | 0.932 | 0.013 | 0.153 | 0.669 |
| Age | −0.005 | 0.99 | −0.122 | 0.28 | 0.078 | 0.588 |
| Children (yes vs. no) | 0.013 | 0.99 | −0.002 | 0.99 | 0.073 | 0.828 |
| Years since diagnosis | 0.158 | 0.181 | 0.135 | 0.181 | 0.088 | 0.413 |
| BRCA-associated cancer (yes vs. no) | −0.234 | 0.588 | −0.134 | 0.669 | −0.045 | 0.99 |
| Lynch-associated cancer (yes vs. no) | −0.298 | 0.266 | −0.023 | 0.99 | −0.197 | 0.508 |
| Anxiety (any vs. none) | −0.534 | 0.266 | −0.472 | 0.413 | −0.523 | 0.266 |
| Depression (any vs. none) | −0.265 | 0.508 | 0.157 | 0.668 | 0.076 | 0.896 |
| Neg/uncertainty | Positive emotions | Positive outlook | Privacy concerns | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Est | p-Value | Est | p-Value | Est | p-Value | Est | p-Value |
| Sex (female vs. male) | 0.328 | 0.18 | 0.012 | 0.953 | 0.875 | 0.003 | 0.344 | 0.18 |
| Age | −0.042 | 0.884 | 0.053 | 0.884 | 0.008 | 0.945 | −0.08 | 0.616 |
| Children (yes vs. no) | 0.22 | 0.442 | −0.032 | 0.945 | 0.196 | 0.495 | −0.087 | 0.93 |
| Years since diagnosis | −0.006 | 0.945 | 0.024 | 0.93 | 0.07 | 0.442 | 0.021 | 0.945 |
| BRCA-associated cancer (yes vs. no) | 0.265 | 0.442 | 0.22 | 0.683 | −0.036 | 0.945 | 0.365 | 0.241 |
| Lynch-associated cancer (yes vs. no) | 0.071 | 0.93 | 0.112 | 0.884 | 0.066 | 0.93 | 0.212 | 0.477 |
| Anxiety (any vs. none) | 0.047 | 0.945 | 0.046 | 0.945 | −0.178 | 0.884 | −0.261 | 0.884 |
| Depression (any vs. none) | 0.984 | 0.003 | −0.089 | 0.93 | −0.024 | 0.945 | 0.664 | 0.18 |
3.2 Sex
Female respondents had significantly higher scores than males on three of the four barrier scales: emotional concerns, insurance concerns, and family concerns. Female patients also had significantly higher scores on the healthcare benefits motivator scale and the positive outlook scale of the FACToR domain than males. These results support hypothesis one regarding family concerns but did not support the anticipated increase in social and interpersonal concerns. We did not have a priori hypotheses regarding health benefits motivator scale nor the positive outlook FACToR scale.
3.3 Age
Younger age was associated with significantly higher scores on three of the four barrier scales, emotional concerns, social and interpersonal concerns, and family concerns. Age was not associated with any of the motivator or FACToR scales.
3.4 Having children
Having children was associated with significantly higher scores on one barrier scale, family concerns, but was not associated with any other scales. This result corresponds to hypothesis three.
3.5 Time from diagnosis
Further time from cancer diagnosis was significantly associated with increased concern about insurance implications of genetic testing. Further time from cancer diagnosis was also significantly associated with increased emotional concerns scale. Time since diagnosis was not associated with any motivator or FACToR scales.
3.6 Cancer type
Having a BRCA-related cancer type was significantly associated only with higher scores on the social and interpersonal concern scale compared to non-BRCA-related cancer types. Having a Lynch-associated cancer was not associated with any of the scales. Hypothesis four related to the social and interpersonal concerns scale result but the results did not support the health benefits part of the hypothesis.
3.7 Mental health
Having a mild-to-moderate depression score on the PHQ-4 was associated with significantly higher scores for three of the four barrier scales (emotional concerns, social and interpersonal concerns, and family concerns) as well as the FACToR scale of negativity/uncertainty compared to those without depression. Presence of anxiety was not associated with any of the scales.
4 DISCUSSION
This cross-sectional study evaluated barriers and motivators to genetic testing among a previously tested population as a pilot study for a larger interventional clinical trial. Patient-level barriers to genetic testing include emotional, insurance, social and interpersonal, and family concerns. Several demographic characteristics (female, younger age, having children, longer time since diagnosis, BRCA-associated cancer type, and depression) were associated with increase report of barriers to genetic testing. Patient-level motivators for genetic testing include internally motivating emotions, health benefits, and interpersonal emotions. Female participants recalled being motivated by the health benefits more than their male counterparts. No other demographic characteristics were associated with motivators.
Females had increased social and interpersonal concerns and family concerns regarding genetic testing compared to males. Studies have shown that women often report these concerns as barriers to testing however there is a lack of research regarding the psychosocial barriers to testing for men Given that males are nearly three times less likely to get testing than females, elucidating the barriers to testing for men is a high priority.33 By having both men and women in our study, we are able to show that there is a significant sex difference in barriers to genetic testing, despite the relatively small percentage of males in our cohort. Further research in a more balanced cohort of men and women is necessary to elucidate the barriers more strongly impacting the decision about genetic testing in men.
Being younger was associated with greater social and interpersonal and family concerns which are consistent with literature reporting increased concerns about family members in young patients receiving genetic testing.34 Younger respondents also expressed more negative emotions as well which has not been previously reported in the literature. While clinicians may suggest speaking to mental health professional regarding an early-onset cancer diagnosis, referral to a genetic counselor may also be needed. Our findings suggest that placing a referral for genetic testing among patients dealing with depression without further psychological support is unlikely to lead to testing.
Having a diagnosis of BRCA-associated cancer was associated with greater social and interpersonal concerns. This is consistent with studies reporting barriers to testing related to the response of people other than the proband.35, 36 Addressing these barriers, as well as providing resources that facilitate the conversation with family members about genetic testing results should be investigated.
A striking finding was that self-reported depressive symptoms were associated with higher report of retrospective barriers for three of the four subscales as well as the negativity/uncertainty subscale of the FACToR. Lerman et al. in 1999 showed that depressive symptoms were a barrier to uptake of genetic testing for Lynch Syndrome patients but there has been a lack of more recent studies evaluating the impact of depression on genetic testing uptake.37 These findings have implications for both pretesting and post-testing counseling. Providers may need to proactively address barriers among those with a history of depression to help them overcome their resistance or ambivalence to genetic testing. Our findings suggest that placing a referral for a patient with depression without further discussion covering barriers and motivators is likely to result in that patient not making an appointment and getting the testing that is clinically indicated. With regard to post-test counseling, the association of depression with the negativity/uncertainty FACToR subscale suggests that clinicians may want to take extra care to prepare patients with a history of depression in the genetic testing referral process as well as discussion of their genetic testing results.
There were several findings for which we had no a priori hypotheses. For example, being female was also associated with increased insurance concerns, a stronger interest in health benefits, and increased positive outlook on the FACToR scale. Given that the majority of the female respondents had breast cancer (62.5%), the latter two findings may be related to greater public awareness of the availability and benefits of genetic testing for breast cancer, however, as we did not ask about this specifically, there may be other underlying reasons for this result.38 We also found that having a more distant cancer diagnosis was associated with increased insurance concerns and greater emotional concerns. This may be explained by the fact that participants who are receiving or have recently completed treatment are less concerned with factors such as future insurance coverage and emotional concerns as they are more preoccupied with more immediate heath concerns such as access to care and treatment costs.39, 40 Further research is needed to understand the reasons why these barriers and motivators were significant for respondents and what role they played in testing decisions. In particular, follow-up surveys for respondents who had a stronger interest in health benefits and had a more positive outlook after testing would guide clinicians in their discussions of testing decisions.
Overall, motivators were not associated with any demographic or clinical variables. The one exception was that females were more likely than males to report an association with anticipated health benefits of testing. Further research is needed to elucidate motivators for genetic testing. Potential future studies include qualitative studies with a demographically diverse patient cohort who have completed genetic testing. We propose that the reasons individuals are motivated to pursue genetic testing may be particularly personal and current research has not captured them appropriately.
The study has several limitations. First is the retrospective manner in which the barrier and motivator questions were assessed. We queried participants to report what they recalled feeling prior to genetic testing for the barrier and motivator questions. It is possible that the experience of getting tested or the timing of their testing (months, years ago) may have impacted their recall. Future prospective studies surveying participants before undergoing genetic testing are needed. Another area to address in future work Is expanding our awareness of additional barriers and motivators. While our survey was informed by literature review and existing measures, it is possible that patients had other barriers/motivators to testing that were not listed in our survey. Another limitation is that the sample population was largely homogeneous; predominantly female (92.5%), white (90.0%), and had BRCA-related cancer (89.2%). Exploring our findings in more demographically and clinically diverse sample is mandated. The data from this study are part of a pilot project and the associated clinical trial is underway with efforts to reach underrepresented populations by enrolling participants from a wide variety of clinical sites across the state of Michigan which serve a more diverse population than the medical center where this study was performed. As a part of this larger clinical trial, we will be gathering information on barriers and motivators as well as testing recommendations and whether or not participants received genetic testing allowing us to further elucidate the correlations between testing status and barriers and motivators.
AUTHOR CONTRIBUTIONS
Erika N. Hanson: Conceptualization (equal); data curation (supporting); project administration (equal); writing – original draft (lead); writing – review and editing (lead). Emerson Delacroix: Project administration (equal); writing – review and editing (equal). Sarah Elizabeth Austin: Writing – review and editing (equal). Grant Carr: Formal analysis (equal); writing – review and editing (supporting). Kelley M Kidwell: Formal analysis (equal); writing – review and editing (supporting). Elizabeth Bacon: Data curation (equal); project administration (supporting); writing – review and editing (supporting). Lynette Hammond Gerido: Writing – review and editing (supporting). Jennifer J Griggs: Funding acquisition (equal); writing – review and editing (equal). Elena M. Stoffel: Funding acquisition (equal); methodology (supporting); writing – review and editing (equal). Ken Resnicow: Conceptualization (equal); funding acquisition (equal); methodology (equal); supervision (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
None.
FUNDING INFORMATION
NIH/NCI U01 CA232827-05: Innovative Approaches to Expand Cancer Genetic Screening and Testing for Patients & Families in a Statewide Oncology Network through Community, State, & Payer Partnerships (PI Stoffel, Griggs, Resnicow).
CONFLICT OF INTEREST STATEMENT
None of the authors have a conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




