Risk factors and prognostic factors for pulmonary large cell neuroendocrine carcinoma with brain metastasis
Xiaoyun Chen and Yedong Huang equally contributed to this work.
Abstract
Background
As the studies regarding the brain metastasis (BM) of pulmonary large cell neuroendocrine carcinoma (LCNEC) are insufficient, the present research aims to describe the risk factors and prognostic factors that are related to cancer-specific survival (CSS) for LCNEC patients with BM.
Methods
The data of LCNEC patients between January 2010 and October 2018 were obtained from the SEER database. Binary logistic regression analyses were utilized to screen the possible risk factors related to BM. Prognostic factors for LCNEC patients with BM were indentified by Cox regression analyses. Moreover, a nomogram was established to predict the 6-, 12-, and 18-month CSS rates. The concordance index (C-index), receiver operating characteristic (ROC) curves and calibration curves were utilized to assess the discrimination and reliability of the model. Clinical decision curves (DCAs) were used to evaluate the clinical benefits and utility of our model.
Results
Totally, 1875 patients were enrolled, with 294 (15.7%) of them having BM at diagnosis. Multivariate logistic regression analyses revealed that patients with age < 65 (odds ratio, OR = 1.564) and N2 staging (OR = 1.775) had a greater chance of developing BM. Age (≥ 65 vs. < 65: hazard ratio, HR = 1.409), T staging (T1 vs. T0: HR = 4.580; T2 vs. T0: HR = 6.008; T3 vs. T0: HR = 7.065; T4 vs. T0: HR = 6.821), N staging (N2 vs. N0: HR = 1.592; N3 vs. N0: HR = 1.654), liver metastasis (HR = 1.410), primary site surgery (HR = 0.581) and chemotherapy (HR = 0.452) were independent prognostic factors for LCNEC patients with BM. A nomogram prediction model was constructed by incorporating these factors. Using the C-index, calibration curves, ROC curves, and DCAs, we found that the clinical prediction model performed well.
Conclusion
We described the risk factors and prognostic factors that were associated with CSS for LCNEC patients with BM. The related nomogram was established and validated to help clinicians formulate more rational and effective treatment strategies.
1 INTRODUCTION
Pulmonary large cell neuroendocrine carcinoma (LCNEC) is a rare type of non-small cell lung cancer (NSCLC), accounting for 1–3% of lung cancer.1-3 LCNEC was reclassified as a subgroup of pulmonary neuroendocrine tumors in 2015,4 which included small cell lung cancer (SCLC), typical carcinoid and atypical carcinoid. LCNEC shows robust malignant behavior and invasive ability.3, 5, 6 About 50% of patients with advanced LCNEC will develop brain metastasis (BM).7, 8 Many patients develop neurological symptoms, which usually lead to serious damage to cognition and quality of life.9-11 Generally, the survival time of such patients is merely a few months.12 The optimal treatment for symptomatic BM are mainly based on local approaches, including surgical resection, stereotactic radiosurgery (SRS) and whole brain radiotherapy (WBRT) which can prevent or slow down the growth of BM.13 The high morbidity and harmfulness of BM make it imperative to explore related risk factors and prognostic factors of BM in LCNEC patients in order that physicians can early identify these patients and offer adequate treatment without delay.
The nomogram model is an intuitive scoring system formed by integrating different variables, which can optimize the prediction accuracy of individuals.14 At present, the model has been widely used in the prognostic evaluation of various diseases, but no research has been completed to construct a prediction model for BM in LCNEC patients. Therefore, data from the Surveillance, Epidemiology, and End Results (SEER) data base were used to explore the risk factors and prognostic factors that were related to cancer-specific survival (CSS) for LCNEC patients with BM. Moreover, a nomogram was established to guide clinical treatment decisions and prognostic judgments.
2 MATERIALS AND METHODS
2.1 Population selection
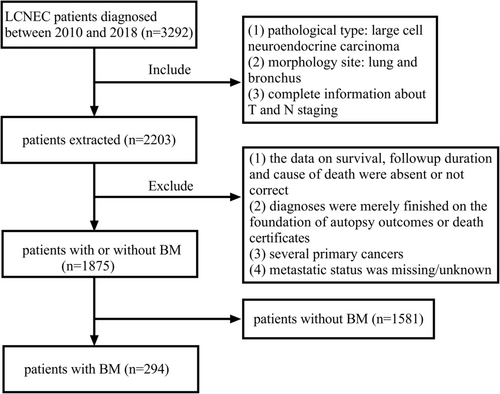
Patient information was acquired from the SEER database. A total of 1875 LCNEC patients between January 2010 and October 2018 were obtained. The inclusion criteria were: (1) pathological type: large cell neuroendocrine carcinoma (ICD-O-3 histologic type: 8013/3); (2) morphology site: lung and bronchus; and (3) complete information about T and N staging. In contrast, the exclusion criteria were (1) the data on survival, follow-up duration and cause of death were absent or not correct; (2) diagnoses were merely finished on the foundation of autopsy outcomes or death certificates; (3) several primary cancers; (4) metastatic status was missing/unknown. According to the presence or absence of BM at diagnosis, we divided the cases with no metastasis of brain into the non-BM group (n = 1581) and those with brain metastasis into the BM group (n = 294). The flow chart of selection is illustrated in Figure 1.
2.2 Data collection
Four variables were extracted according to demographic characteristics, including age at diagnosis(< 65, ≥ 65 years), gender (males or females), race (white, black, and others), and marital status at diagnosis (married or unmarried). Eight variables were extracted according to cancer characteristics, including primary sites (main bronchus, upper lobe, middle lobe, lower lobe, and others), laterality (left, right, and unknown), T staging (T0, T1, T2, T3, and T4), N staging (N0, N1, N2 and N3), bone metastases (no or yes), liver metastases (no or yes), lung metastases (no or yes) and AJCC seventh edition staging (I, II, III, IV and unknown). Three variables were extracted according to treatment regimens, including primary site surgery (no/unknown or yes), radiotheraphy (no/unknown or yes) and chemotherapy (no/unknown or yes). Cancer specific survival (CSS) is considered to be the time from diagnosis to death due to LCNEC. In this article, CSS was utilized as the main endpoint.
2.3 Statistical analyses
The basic clinicopathological data of each group were analyzed by the chi-square test to show the differences between the non-BM and BM groups, which were displayed as frequencies and proportions (%). Univariate logistic regression analyses were utilized to screen the possible risk factors related to BM. The variates with p < 0.05 in the univariate logistic regression analyses were included in multivariate logistic regression analyses. Survival differences were evaluated by the Kaplan–Meier method and Log-rank test. The prognostic factors with significant differences related to CSS in univariate Cox regression analyses were verified by multivariate Cox regression analyses. The nomogram was then built by the ‘rms’ package in the R software based on independent prognostic factors. The discrimination degree of the nomogram was assessed via the concordance index (C-index) and receiver operating characteristic (ROC) curves. At the same time, the calibration degree of the nomogram was tested by drawing calibration curves to ensure the accuracy and reliability. Additionally, clinical decision curves (DCAs) were used to evaluate the clinical benefits and utility of our model. This study used the SPSS Statistics software 25.0 to perform the chi-square test, univariate logistic regression analysis, multivariate logistic regression analysis, survival analysis, univariate Cox regression analysis and multivariate Cox regression analysis. Calibration curves, ROC curves and DCAs were drawn using the R language software 4.1.3. p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Baseline features
Totally, 1875 patients were enrolled, and 294 (15.7%) of them were in the BM group while 1581 (84.3%) of them were in the non-BM group. The BM group was significantly younger (51.7% vs. 39.5%) than the non-BM group (p < 0.001). Statistical analysis revealed that remarkable diversities existed in T and N staging between BM and non-BM patients (p < 0.001). What's more, the BM group had more bone metastasis (22.8% vs. 12.8%), liver metastasis (23.5% vs. 14.7%) and lung metastasis (17.0% vs. 10.4%) compared with non-BM group (p < 0.001). In terms of treatment, BM patients had less primary site surgery (7.8% vs. 43.1%, p < 0.001) and more radiotherapy (78.2% vs. 32.7%, p < 0.001). Additionally, the distribution of gender, race, marriage, primary site, laterality and chemotherapy was not associated with statistically significant outcomes (p > 0.05). The baseline features are presented in Table 1.
| Variable | BM, n (%) n = 294 (15.7) | No BM, n (%) n = 1581 (84.3) | Total, n (%) n = 1875 | p-value |
|---|---|---|---|---|
| Age (years) | <0.001 | |||
| < 65 | 152 (51.7) | 624 (39.5) | 776 (41.4) | |
| ≥ 65 | 142(48.3) | 957 (60.5) | 1099 (58.6) | |
| Gender | 0.334 | |||
| Female | 144 (49.0) | 726 (45.9) | 870 (43.2) | |
| Male | 150 (51.0) | 855(54.1) | 1005 (53.6) | |
| Race | 0.535 | |||
| White | 252 (85.7) | 1314 (83.1) | 1566 (83.5) | |
| Black | 32 (10.9) | 207 (13.1) | 239 (12.7) | |
| Others | 10 (3.4) | 60 (3.8) | 70 (3.7) | |
| Marriage | 0.487 | |||
| Married | 156 (53.1) | 804 (50.9) | 960 (51.2) | |
| Unmarried | 138 (46.9) | 777 (49.1) | 915 (48.8) | |
| Primary Site | 0.198 | |||
| Main bronchus | 14 (4.8) | 65 (4.1) | 79 (4.2) | |
| Upper lobe | 156 (53.1) | 912 (57.7) | 1068 (57.0) | |
| Middle lobe | 12 (4.1) | 63 (4.0) | 75 (4.0) | |
| Lower lobe | 74 (25.2) | 404 (25.6) | 478 (25.5) | |
| Others | 38 (12.9) | 137 (8.7) | 175 (9.3) | |
| Laterality | 0.059 | |||
| Left | 112 (38.1) | 642 (40.6) | 754 (40.2) | |
| Right | 170 (57.8) | 909 (57.5) | 1079 (57.5) | |
| Unknown | 12 (4.1) | 30 (1.9) | 42 (2.2) | |
| T stage | <0.001 | |||
| T0 | 5 (1.7) | 13 (0.8) | 18 (1.0) | |
| T1 | 46 (15.6) | 444 (28.1) | 490 (26.1) | |
| T2 | 82 (27.9) | 487 (30.8) | 569 (30.3) | |
| T3 | 80 (27.2) | 325 (20.6) | 405 (21.6) | |
| T4 | 81 (27.6) | 312 (19.7) | 393 (21.0) | |
| N stage | <0.001 | |||
| N0 | 90 (30.6) | 726 (45.9) | 816 (43.5) | |
| N1 | 32 (10.9) | 151 (9.6) | 183 (9.8) | |
| N2 | 127 (43.2) | 493 (31.2) | 620 (33.1) | |
| N3 | 45 (15.3) | 211 (13.3) | 256 (13.7) | |
| Bone Metastasis | <0.001 | |||
| No | 227 (77.2) | 1378 (87.2) | 1605 (85.6) | |
| Yes | 67 (22.8) | 203 (12.8) | 270 (14.4) | |
| Liver Metastasis | <0.001 | |||
| No | 225 (76.5) | 1349 (85.3) | 1574 (83.9) | |
| Yes | 69 (23.5) | 232 (14.7) | 301 (16.1) | |
| Lung Metastasis | 0.001 | |||
| No | 244 (83.0) | 1416 (89.6) | 1660 (88.5) | |
| Yes | 50 (17.0) | 165 (10.4) | 215 (11.5) | |
| Stage | - | |||
| Unknown | 0 (0.0) | 96 (6.1) | 96 (5.1) | |
| I | 0 (0.0) | 329 (20.8) | 329 (17.5) | |
| II | 0 (0.0) | 146 (9.2) | 146 (7.8) | |
| III | 0 (0.0) | 239 (15.1) | 239 (12.7) | |
| IV | 294 (100.0) | 771 (48.8) | 1065 (56.8) | |
| Primary site surgery | <0.001 | |||
| No/Unknown | 271 (92.2) | 899 (56.9) | 1170 (62.4) | |
| Yes | 23 (7.8) | 682 (43.1) | 705 (37.6) | |
| Radiotherapy | <0.001 | |||
| No/Unknown | 64 (21.8) | 1064 (67.3) | 1128 (60.2) | |
| Yes | 230 (78.2) | 517 (32.7) | 747 (39.8) | |
| Chemotherapy | 0.071 | |||
| No/Unknown | 126 (42.9) | 768 (48.6) | 894 (47.7) | |
| Yes | 168 (57.1) | 813 (51.4) | 981 (52.3) |
- Abbreviation: BM, brain metastasis.
Risk factors for developing BM.
The clinical characteristics of age, gender, race, marriage, primary site, T staging and N staging were studied via univariable logistic regression analyses. Then the risk factors that might be related to BM were involved in multivariable logistic regression analyses. Eventually, the outcomes revealed that patients with younger age (OR = 1.564, 95% CI: 1.215–2.013) and N2 staging (OR = 1.775, 95% CI: 1.306–2.412) had a higher incidence of BM (p < 0.05), while those with T1 staging (OR = 0.329, 95% CI: 0.110–0.980) had a lower incidence of BM (p > 0.05). The details are displayed in Table 2.
| Variable | Univariate logistic | Multivariate logistic | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years) | ||||
| ≥ 65 | Reference | Reference | ||
| < 65 | 1.642 (1.279–2.108) | <0.001 | 1.564 (1.215–2.013) | 0.001 |
| Gender | ||||
| Female | Reference | |||
| Male | 0.885 (0.689–1.135) | 0.334 | ||
| Race | ||||
| White | Reference | |||
| Black | 0.806 (0.543–1.198) | 0.286 | ||
| Others | 0.869 (0.439–1.720) | 0.687 | ||
| Marriage | ||||
| Married | Reference | |||
| Unmarried | 0.915 (0.713–1.175) | 0.487 | ||
| Primary site | ||||
| Main bronchus | Reference | |||
| Upper lobe | 0.794 (0.435–1.450) | 0.453 | ||
| Middle lobe | 0.884 (0.380–2.059) | 0.776 | ||
| Lower lobe | 0.850 (0.454–1.594) | 0.613 | ||
| Others | 1.288 (0.652–2.542) | 0.466 | ||
| T stage | ||||
| T0 | Reference | Reference | ||
| T1 | 0.269 (0.092–0.789) | 0.017 | 0.329 (0.110–0.980) | 0.046 |
| T2 | 0.438 (0.152–1.261) | 0.126 | 0.498 (0.171–1.452) | 0.202 |
| T3 | 0.640 (0.222–1.847) | 0.409 | 0.703 (0.241–2.050) | 0.518 |
| T4 | 0.675 (0.234–1.948) | 0.467 | 0.676 (0.232–1.971) | 0.474 |
| N stage | ||||
| N0 | Reference | Reference | ||
| N1 | 1.709 (1.101–2.654) | 0.017 | 1.546 (0.992–2.411) | 0.054 |
| N2 | 2.078 (1.550–2.786) | <0.001 | 1.775 (1.306–2.412) | <0.001 |
| N3 | 1.720 (1.166–2.539) | 0.006 | 1.308 (0.867–1.972) | 0.200 |
- Abbreviations: CI, confidence interval; OR, odds ratio.
3.2 Survival outcomes and prognostic factors
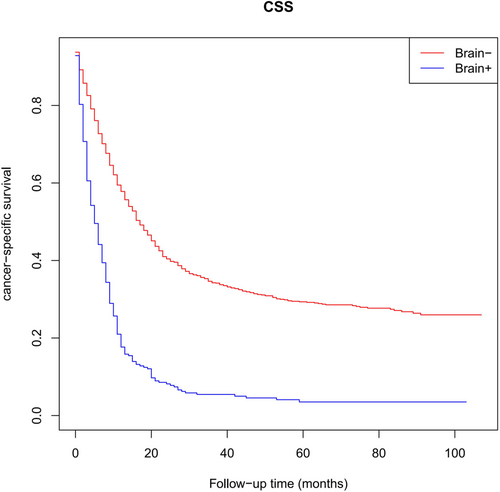
As presented in Figure 2, survival analyses revealed that BM patients had significantly lower CSS compared with non-BM patients (5 months vs. 17 months, p < 0.001). Therefore, LCNEC patients with BM often exhibited unfavorable outcomes. The concrete outcomes of the median survival are presented in Table 3. In univariable Cox regression analyses, age, T staging, N staging, bone metastasis, liver metastasis, primary site surgery, radiotheraphy and chemotherapy were the predictors of CSS for LCNEC patients with BM (p < 0.05). Subsequently, multivariable Cox regression analyses indicated that an increasing age (hazard ratio, HR = 1.409), T staging > T0 (T1 vs. T0: HR = 4.580; T2 vs. T0: HR = 6.008; T3 vs. T0: HR = 7.065; T4 vs. T0: HR = 6.821), N staging > N1 (N2 vs. N0: HR = 1.592; N3 vs. N0: HR = 1.654) and liver metastases (HR = 1.410) were independent poor prognostic factors (HR >1, p < 0.05). On the other hand, primary site surgery (HR = 0.581) and chemotherapy (HR = 0.452) were independent favorable prognostic factors (HR <1, p < 0.05). The complete outcomes are presented in Table 4.
| Patients, N Median CSS 95% CI, Months | ||
|---|---|---|
| No BM | 1581 | 17 (15.314–18.686) |
| BM | 294 | 5 (3.944–6.056) |
| p-value | <0.001 | |
- Abbreviations: BM, brain metastasis; CI, confidence interval; CSS, cancer-specific survival.
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (years) | ||||
| < 65 | Reference | Reference | ||
| ≥ 65 | 1.483 (1.162–1.894) | 0.002 | 1.409 (1.092–1.819) | 0.008 |
| Gender | ||||
| Female | Reference | |||
| Male | 1.102 (0.868–1.400) | 0.424 | ||
| Race | ||||
| White | Reference | |||
| Black | 0.785 (0.536–1.150) | 0.214 | ||
| Others | 0.766 (0.406–1.445) | 0.410 | ||
| Marriage | ||||
| Married | Reference | |||
| Unmarried | 1.171 (0.922–1.488) | 0.196 | ||
| Primary site | ||||
| Main bronchus | Reference | |||
| Upper lobe | 1.117 (0.644–1.936) | 0.694 | ||
| Middle lobe | 0.939 (0.425–2.073) | 0.876 | ||
| Lower lobe | 1.372 (0.771–2.441) | 0.282 | ||
| Others | 1.059 (0.563–1.991) | 0.859 | ||
| T stage | ||||
| T0 | Reference | Reference | ||
| T1 | 6.017 (1.449–24.976) | 0.013 | 4.580 (1.093–19.197) | 0.037 |
| T2 | 6.247 (1.527–25.566) | 0.011 | 6.008 (1.460–24.719) | 0.013 |
| T3 | 7.369 (1.802–30.145) | 0.005 | 7.065 (1.717–29.072) | 0.007 |
| T4 | 8.506 (2.078–34.821) | 0.003 | 6.821 (1.655–28.118) | 0.008 |
| N stage | ||||
| N0 | Reference | Reference | ||
| N1 | 1.429 (0.941–2.172) | 0.094 | 1.388 (0.902–2.136) | 0.136 |
| N2 | 1.622 (1.214–2.167) | 0.001 | 1.592 (1.168–2.170) | 0.003 |
| N3 | 1.683 (1.159–2.443) | 0.006 | 1.654 (1.114–2.455) | 0.013 |
| Bone metastasis | ||||
| No | Reference | Reference | ||
| Yes | 1.727 (1.298–2.299) | <0.001 | 1.190 (0.865–1.638) | 0.285 |
| Liver metastasis | ||||
| No | Reference | Reference | ||
| Yes | 1.490 (1.124–1.937) | 0.005 | 1.410 (1.026–1.937) | 0.034 |
| Lung metastasis | ||||
| No | Reference | |||
| Yes | 1.314 (0.958–1.802) | 0.090 | ||
| Primary site surgery | ||||
| No/Unknown | Reference | Reference | ||
| Yes | 0.510 (0.323–0.807) | 0.004 | 0.581 (0.360–0.937) | 0.026 |
| Radiotherapy | ||||
| No/Unknown | Reference | Reference | ||
| Yes | 0.617 (0.462–0.823) | 0.001 | 0.779 (0.580–1.046) | 0.097 |
| Chemotherapy | ||||
| No/Unknown | Reference | Reference | ||
| Yes | 0.514 (0.403–0.657) | <0.001 | 0.452 (0.346–0.591) | <0.001 |
- Abbreviations: CI, confidence interval; HR, hazard ratio.
3.3 Prognostic nomogram construction and validation
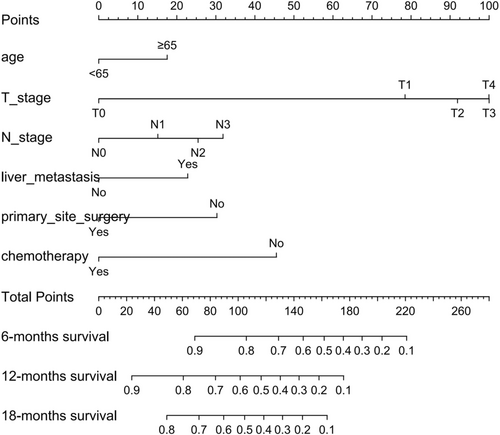
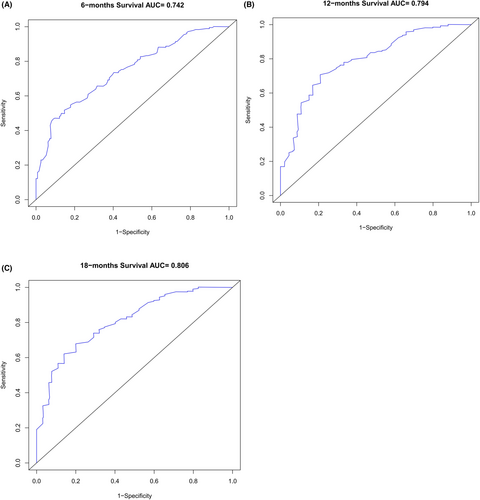
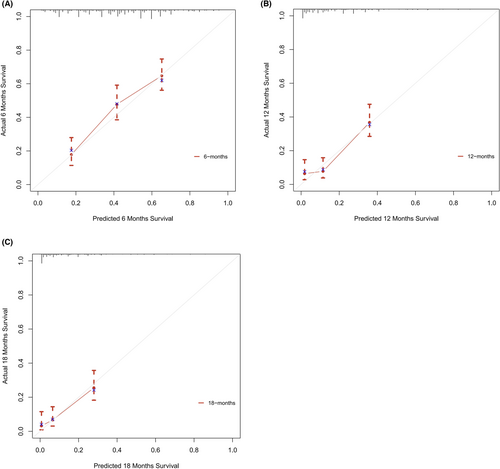
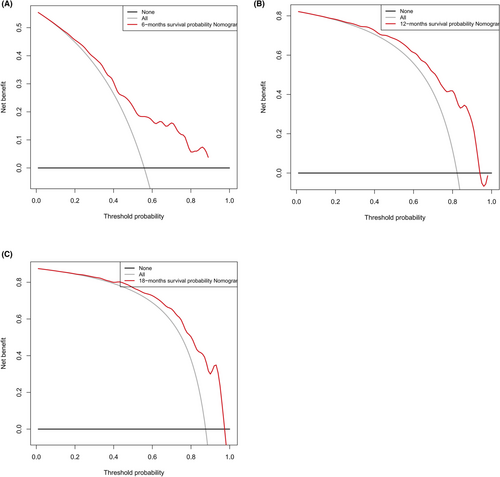
Based on the 6 aforementioned prognostic factors, we established a CSS nomogram to forecast the survival probability of LCNEC patients with BM, as shown in Figure 3. The scoring for every variable was added together to obtain an overall score to predicted the 6-, 12-, and 18-month survival probability for each patient. It was interesting that T staging had the greatest impact on prognoses, followed by N staging and chemotherapy. The C-index of the nomogram was 0.712 (95% CI: 0.677–0.747). The area under ROC curves (AUC) for predicting the 6-month, 12-month, and 18-month CSS rates was 0.727, 0.776 and 0.78 (Figure 4), respectively, showing good predictive accuracy. At the same time, the calibration curves for the 6-, 12-, and 18-month CSS rates were all close to the ideal 45° reference line (Figure 5), suggesting a high consistency between the predicted and actual values. DCA analyses revealed that the predictive model displayed satisfactory clinical benefits and utility (Figure 6).
4 DISCUSSION
LCNEC is a highly aggressive malignancy prone to brain metastasis.7, 8 Currently, this is the first large population study for LCNEC patients with BM. Our findings showed that 15.7% of LCNEC patients developed BM. Younger age and N2 staging were associated with a greater chance of developing BM. In addition, age, T staging, N staging, liver metastasis, primary site surgery and chemotherapy were independent prognostic factors for LCNEC patients with BM. The related prognostic model based on these factors can guide clinical treatment decisions and prognostic judgments.
Substantial literatures indicated that younger NSCLC patients were more prone to brain metastasis.15, 16 Consistent with these reports, our findings showed that patients with younger age had a higher incidence of BM. It is considered that younger patients often live longer, hence they have more time to develop BM. Besides, as lymph nodes are usually the first stop for cancer cells to metastasize and cancer cells can also spread through the lymphatics into the brain,17 we discovered that LCNEC patients with BM tended to have more N2 staging than non-BM patients. Thereby, inhibiting lymphatic spread may be an effective method to prevent distant metastasis.18 Experiments have shown that lymphatic vessels can provide a diffusion pathway in mouse models, but whether this form of cancer cell spread exists in humans needs to be verified.19, 20
In terms of prognostic factors in LCNEC patients with BM, several previous studies suggested that age > 65 years was related to poor prognosis.21-23 Our study also observed significant differences regarding the prognosis between older patients and younger patients. Most elderly patients have underlying diseases, such as hypertension, diabetes and other chronic diseases. Some of them are bedridden for a long time, which often results in poor physical status. In the traditional TNM staging system, lung cancer patients will survive longer with earlier T and N staging, which is supported by our prediction model. Our study identified T staging > T0 as an independent poor prognostic factor. The increase in T staging means an increase in tumor volume, which is a manifestation of further malignant tumor progression. What's more, we discovered that patients with N2 and N3 staging had lower survival rates than patients with N0 staging. We speculate that lymphatics may be the way for tumor cells to spread, which aggravates the survival of patients. Consistent with the finding of Liu CF et al,13 our study also found that liver metastases in LCNEC patients with BM shortened the survival time. The occurrence of liver metastases often indicates that the tumor is more aggressive, hence the prognosis is relatively poorer.
Nowadays, the standard therapeutic regimen for LCNEC patients with BM is still uncertain.8, 24, 25 Our study hold that primary site surgery treatment is an important component of the comprehensive treatment in advanced pulmonary LCNEC, which is consistent with the results of several previous studies.26-28 If primary site surgery is not possible, chemotherapy can be considered. The pathological features of LCNEC are similar to SCLC, whereas it is classified as NSCLC. Physicians can choose the NSCLC regimen and SCLC regimen. Several studies have shown that the SCLC regimen is more effective. Four to six cycles of etoposide in combination with either cisplatin or carboplatin are usually recommended for advanced LCNEC patients.29-31 However, the research of Nicole Roberts et al showed no statistically significant difference between the two chemotherapy regimens.32 During the last decade, targeted therapy for oncogene-dependent diseases and immunotherapy for patients with PD-L1 positive cancers have transformed the treatment options for NSCLC. For pulmonary LCNEC, target gene mutations are predominantly found in mixed forms of LCNEC-adenocarcinoma, while ‘pure’ LCNEC target gene mutations are rare. It has been reported that LCNEC patients with EGFR mutations may benefit from gefitinib treatment.33 For patients with ALK rearrangements detected in LCNEC, the efficacy of TKI therapy is conflicting.34, 35 Although PI3K/AKT/mTOR pathway alterations are frequently detected in LCNEC,36, 37 unfortunately, no effective targeted drugs have been developed. Delta-like protein 3 is an inhibitory Notch ligand expressed in approximately 65% of LCNEC, which provides an excellent target for the development of antibody–drug conjugates (ADCs). Compared with SCLC and low-grade neuroendocrine tumors, LCNEC has a higher level of PD-L1 expression.38, 39 In a case report of Chauhan et al, 3 LCNEC patients with unsatisfactory platinum-based chemotherapy results were treated with nivolumab immunotherapy and achieved objective response or stable disease.40 An advanced LCNEC patient with a high tumor mutation burden level was reported in another case, who achieved a complete response to nivolumab treatment.41 These studies provided insights into the efficacy of immunotherapy. To date, targeted therapy or immunotherapy are not indicated for patients with LCNEC. Clinical trials involving LCNEC are strongly recommended in order to determine an optimal treatment strategy and correlate biomolecular properties with the promising involvement of new therapeutic options.
As a visual tool for cancer prognostic assessment, the nomogram has been used to evaluate the prognosis of LCNEC patients and demonstrates good predictive value. However, the model that can forecast the prognosis of LCNEC patients with BM has not been developed yet. In this study, age, T staging, N staging, liver metastases, primary site surgery and chemotherapy were incorporated into the prediction model according to the outcomes of multivariable Cox regression analyses, and a nomogram was established. The C-index and AUC were high and the calibration curves for the 6-, 12-, and 18-month CSS rates showed good consistency, which revealed that the model had satisfactory discrimination and calibration. Therefore, it has certain value for clinicians to judge the prognosis of LCNEC patients with BM.
However, there are some deficiencies. First, as our study utilized retrospective data from the SEER database, the results require the substantiation from more prospective trials. Second, the data on smoking status, comorbidities, performance scores, targeted therapies and immunotherapies were absent due to the constraints of the SEER database, which might affect survival results. Third, although the predictive model established in this study showed good accuracy and clinical applicability, patients were not divided into modeling groups and validation groups for model verification because the number of cases in some subgroups was too small. Eventually, as we did not have sufficient data from other sources due to the rarity of BM, external validation is required to realize further corroboration in the future.
5 CONCLUSION
Our study described the risk factors and prognostic factors that were associated with CSS for LCNEC patients with BM. The related nomogram was established and validated to help clinicians formulate more rational and effective treatment strategies.
AUTHOR CONTRIBUTIONS
Xiaoyun Chen: Writing – original draft (equal). Yedong Huang: Writing – original draft (equal). Fangrong Chen: Formal analysis (equal). Hui She: Writing – review and editing (equal). Xiangqi Chen: Writing – review and editing (equal).
ACKNOWLEDGMENTS
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in providing high-quality open resources for researchers.
FUNDING INFORMATION
This research was supported by the Science Technology Innovation Joint Project Foundation of Fujian Province (2018Y9038) and Fujian provincial health technology project (2020CXB016).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




