Front-line treatment efficacy and clinical outcomes of elderly patients with multiple myeloma in a real-world setting: A multicenter retrospective study in China
Li Bao and Ai-Jun Liu contributed equally and both were considered the first authors.
Abstract
Background
The use of proteasome inhibitors (PIs), new immune modulators (IMiDs), and other new drugs, as well as high-dose chemotherapy combined with autologous stem cell transplantation has considerably improved the survival of young patients with multiple myeloma (MM). However, the improvement in survival among elderly patients remains insufficient. Optimal treatment recommendation models for elderly patients with MM have not been developed especially there are quite few study in the real world.
Methods
We retrospectively analyzed the treatment patterns and outcomes of 328 Chinese patients (≥65 years) with MM in a real-world setting. Patients were divided into three groups according to induction regimens.
Results
The median age of the cohort was 70 (65–86) years. The patients were divided into group 1 (PIs based regimens, n = 218), group 2 (IMiDs based regimens, n = 48) and group 3 (PIs + IMiDs, n = 62). Induction regimens in group 3 produced higher overall response rate than group 1 and 2 (85.42% vs. 71.08% vs. 66.67%, p = 0.016). The median follow-up of the cohort was 30 (interquartile range [IQR] 18–36) months. For the entire cohort median progression-free survival (PFS) was 26 (IQR 12.00–42.89) months and overall survival (OS) was 60 (IQR 40.00–67.20) months. The PFS were not significantly different among the three groups (28 months vs. 18 months vs. 26 months, p = 0.182). So were the OS (60 months vs. 59 months vs. not reached, p = 0.067). Multivariate analysis revealed that age >70 year, frailty status (Geriatric vulnerability score), induction efficacy < partial remission, and no maintenance treatment were independent poor prognostic factors for OS.
Conclusion
Front-line induction regimens combining PIs and IMiDs developed more deep response than single PI or IMiD based regimens. Maintenance treatment can further improve the clinical outcome in elderly MM patients in real-world setting.
1 INTRODUCTION
Multiple myeloma (MM) is a malignant clonal plasma cell disorder that is currently considered incurable. The median age at diagnosis is approximately 70 years and the incidence increases with age.1, 2 The use of proteasome inhibitors (PIs), immune modulators (IMiDs), and other novel drugs, as well as high-dose chemotherapy combined with autologous stem cell transplantation, has considerably improved the survival of patients with MM. Although with continuing advances in treatments the survival rates are improving, age at diagnosis remains an important determinant of outcome.3
In general, patients with newly diagnosed MM are classified into two groups: transplant-eligible and transplant-ineligible and are treated by different strategies. For the transplant-eligible patients a three-drug combination induction therapy followed by consolidation with transplantation and then maintenance treatment have been considered as standard treatment pattern.4 For transplant-ineligible patients, the treatment is widely heterogenous. The dividing line betwen induction and maintenance is not clear depending on physicians' discretion in different areas.
Over the past 20 years, treatment of transplant-ineligible MM patients evolved from melphalan/prednisone to IMiD or PI based regimens, and then to a three-drug combination based on backbone of lenalidomide/dexamethasone. More recently, anti-CD38 mAb therapy has also been incorporated into these regimens. In addition, moving from fixed duration treatment to continuous therapy also contributed to the improved survival outcomes. Nevertheless, for elderly MM patients, the optimal model of treatment is not standardized yet.
So far, the therapeutic experience with MM has dominated by data from clinical trials in which the elderly patients were often excluded due to poor physical status and comorbidities. So the evidence of elderly patients from the real-world practice is even more noteworthy. Up till now quite few relevant real-world studies have been conducted on elderly subpopulation with MM, mostly from Europe and North America.5-7 There is a lack of data on treatment patterns and survival of elderly Asian patients. In China, novel agents such as bortezomib, ixazomib, and lenalidomide, have been reimbursed by medical insurance system after 2016, which brought advantages of clinical availability. Before that, the utilization of novel agents was only approximately 50%8. Therefore, dramatic changes could have developed during past 5 year. In this study, we analyzed the front-line treatment models in elderly MM patients from four major medical centers, compared the impact of induction regimens and maintenance therapy on clincial outcomes, and discussed the current problems in real-world practice, providing a reference to further improve the survival of elderly MM patients in the future.
2 MATERIALS AND METHODS
2.1 Study design and patients
This was a retrospective cohort study. Data of 362 newly diagnosed elderly MM patients (age ≥65 years) were collected from four medical centers in China between January 2016 and December 2020, and all patients met the International Myeloma Working Group (IMWG) diagnostic criteria.9 Patients with smoldering MM, plasma cell leukeima or solitary plasmacytoma were excluded. Only a few patients were treated with conventional chemotherapy so that this minor group was excluded. The four centers were Beijing Jishuitan Hospital, Beijing Chaoyang Hospital, Peking University First Hospital and Peking Union Medical College Hospital. The retrospective follow-up period was till December 2021. The protocol was approved by the institutional medical ethics committee and informed consent were assigned before treatment (Ethical approval number JST201907-04).
2.2 Research variables
For each patient, data regarding age, gender, type of M protein, Hemoglobin (HGB), calcium, dehydrogenase (lactate dehydrogenase [LDH]) and bone marrow plasma cell perccentage as well as complications were collected as base-line characteristics. The albumin (ALB) and beta 2-microglobulin (β2-MG) were recorded for International Staging System (ISS). Estimated glomerular filtration rate (eGFR) was calculated. Geriatric vulnerability score (GA score)10 was used to evaluate the frailty status. High-risk chromosomal abnormalities11 referred to any one of amplification of 1q21, 17P deletion, t (4;14),t (14;16), or t (14;20) detected by fluorescence in situ hybridization (FISH).
2.3 Treatment process
Patients were divided into three groups according to the front-line induction therapies including PIs (bortezomib or ixazomib, group 1), regimens including IMiDs (lenalidomide or thalidomide, group 2), and regimens combining PIs and IMiDs (group 3). None of the patients underwent transplantation. The maintenance treatment after front-line induction comprised IMiDs or PIs or both. The optimal response12 after induction was reflected in partial response (PR), very good partial response (VGPR) and complete response (CR). The overall response rate (ORR) = PR + VGPR+CR. Efficacy PR or better were regarded as effective. Patents whose response were less than PR were considered ineffective and administrated the second-line regimens.
2.4 Follow-up
Till December 2021, clinical outcomes were assessed using progression-free survival (PFS) and overall survival (OS) with a median follow-up of 30 months (interquartile range [IQR] 18–36). PFS refers to the time from the beginning of therapy to the first occurrence of disease progression or death. OS refers to the time from diagnosis to death, regardless of causes.
2.5 Statistical methods and data analyses
Patients were grouped by front-line induction regimens. The clinical characteristics of our cohort were done using descriptive statistics and non-normally distributed data in each group were analyzed using the Mann–Whitney U test. The comparison of interest defined a response priority between PIs + IMiDs regimen versus PIs and PIs + IMiDs versus IMiDs. Logistic regression was used to evaluate the association between induction response and the front-line regimens (VGPR and better response vs. other). In order to control confounding factors, we conducted multivariable analysis adjusted for age, GA score, ALB, ISS stage, eGFR and FISH (results reported as adjusted OR and HR). PFS and OS were analyzed using Kaplan–Meier survival curves and the log-rank test. Multivariate analysis of risk factors for PFS and OS was performed using Cox modeling. Two-tailed p values <0.05 were considered as statistically significant. SPSS statistical software version 20.0 (IBM Corp.) was used in our analysis.
3 RESULTS
3.1 Patient baseline characteristics
3.1.1 General description of the sample
Of the 362 patients, 24 (10 males and 14 females) abandoned treatment for economic reason, with a median age of 71 (65–85) years. Ten patients (six males and four females) treated with traditional chemotherapy was excluded. In total, 328 patients were included, and their baseline data were listed in Table 1. Briefly, there were 177 males and 151 females with median age of 70 (65–86) years. The detailed age distribution was 172 patients (52.43%) aged 65–70 years, 130 (39.63%) aged 71–79 years, and 26 (7.93%) aged ≥80 years. There were 59.76% of patients in ISS stage III and 36.89% in Revised International Scoring System (R-ISS) stage III. FISH high-risk percentage was 18.90% and GA score frail patients accounted for less than 1/3 (22.26%).
| Parameter | Median value (range) or number (%) |
|---|---|
| Age, years old | |
| Median(range) | 70 (65–86) |
| 65–70 | 172 (52.44%) |
| 71–79 | 130 (39.63%) |
| ≥80 | 26 (7.93%) |
| Male | 177 (53.96%) |
| Female | 151 (46.04%) |
| M protein | |
| IgG | 146 (44.51%) |
| IgA | 77 (23.48%) |
| IgD | 16 (4.88%) |
| Light chain | 61 (18.60%) |
| Non-secreting | 18 (5.49%) |
| Missing | 10 (3.04%) |
| DS stage | |
| I | 17 (5.18%) |
| II | 41 (12.50%) |
| III | 264 (80.49%) |
| Missing | 6 (1.83%) |
| SCr (mol/L) | 86.20 (31–1370) |
| Ca (mmol/L) | 2.32 (1.11–4.73) |
| HGB (g/L) | 97 (42–158) |
| LDH (IU/L) | 173 (14–2048) |
| β 2-MG (mg/L) | 5.58 (0.79–58.06) |
| ALB (g/L) | 33.15 (12.50–47.90) |
| ISS stage | |
| I | 36 (10.97%) |
| II | 81 (24.70%) |
| III | 196 (59.76%) |
| Missing | 15 (4.57%) |
| R-ISS stage | |
| I | 24 (7.32%) |
| II | 146 (44.51%) |
| III | 121 (36.89%) |
| Missing | 37 (11.28%) |
| BMPC percentage (%) | 24.25 (0–98) |
| FISH | |
| Standard-risk | 203 (61.89%) |
| High-risk | 62 (18.90%) |
| Missing | 63 (19.21%) |
| GA score | |
| Fit | 114 (34.76%) |
| Intermediate fit | 17 (5.18%) |
| Frail | 73 (22.26%) |
| Missing | 124 (37.80%) |
- Abbreviations: ALB, serum albumin; BMPC, bone marrow plasma cells; Ca, corrected serum calcium; FISH, fluorescence in situ hybridization; GA score, geriatric vulnerability score; HGB, hemoglobin; ISS, International Scoring System; LDH, lactate dehydrogenase; R-ISS, Revised International Scoring System; SCr, serum creatinine; β2-MG, beta 2-microglobulin.
3.1.2 Baseline difference between the three groups
As shown in Table 2, the proportions of hypoproteinemia (ALB <30 g/L), kidney failure (eGFR ≤40 ml/min), FISH high-risk, or GA score indicating fitness in group 3 were higher than those in group 1 and 2 (p < 0.05). There were no significant differences in other characteristics including age, gender, β2-MG levels, marrow plasma cell percentage and the proportion of hypercalcemia (≥2.75 mmol/L), high LDH (≥250 IU/L), anemia (HGB <100 g/L), and ISS or R-ISS stage distribution among the three groups (p > 0.05).
| Total (n = 328) | PIs (Group 1, n = 218) | IMiDs (Group 2, n = 48) | PIs + IMiDs (Group 3, n = 62) | p value | |
|---|---|---|---|---|---|
| Age (year) | 70 (65–86) | 70 (65–86) | 72 (65–86) | 69 (65–82) | 0.068 |
| Male | 177 (53.96%) | 122 (55.96%) | 23 (47.92%) | 32 (51.61%) | 0.502 |
| Female | 151 (46.04%) | 96 (44.04%) | 25 (52.08%) | 30 (48.39%) | |
| Calcium ≥2.75 mmol/L | 36 (10.98%) | 29 (13.30%) | 3 (6.25%) | 4 (6.45%) | 0.196 |
| eGFR ≤40 ml/min | 64 (19.51%) | 41 (18.80%) | 7 (14.58%) | 16 (25.80%) | 0.043 |
| LDH ≥250 IU/L | 51 (15.55%) | 37 (16.97%) | 5 (10.42%) | 9 (14.52%) | 0.355 |
| HGB <100 g/L | 187 (57.01%) | 128 (58.72%) | 26 (54.17%) | 33 (53.23%) | 0.831 |
| β2-MG (mg/L) | 5.58 (0.79–58.06) | 5.51 (1.18–58.06) | 5.01 (2.10–51.00) | 5.76 (0.79–39.93) | 0.618 |
| ALB <30 (g/L) | 86 (26.22%) | 48 (22.02%) | 10 (20.83%) | 28 (45.16%) | 0.003 |
| BMPC (%) | 24.25 (0–98) | 23.75 (0–98) | 29.50 (0–88) | 28.50 (0–93) | 0.115 |
| ISS stage | |||||
| I | 36 (10.96%) | 25 (11.47%) | 7 (14.58%) | 4 (6.45%) | 0.191 |
| II | 81 (24.70%) | 57 (26.15%) | 12 (25.00%) | 12 (19.35%) | |
| III | 196 (59.76%) | 128 (58.71%) | 26 (54.17%) | 42 (67.74%) | |
| Missing | 15 (4.57%) | 8 (3.67%) | 3 (6.25%) | 4 (6.45%) | |
| R-ISS stage | 0.147 | ||||
| I | 24 (7.32%) | 17 (7.80%) | 6 (12.50%) | 1 (1.60%) | |
| II | 146 (44.51%) | 97 (44.50%) | 22 (45.80%) | 27 (43.50%) | |
| III | 121 (36.89%) | 80 (36.70%) | 14 (29.20%) | 27 (43.50%) | |
| Missing | 37 (11.28%) | 24 (11.00%) | 6 (12.50%) | 7 (11.30%) | |
| GA score | 0.001 | ||||
| Fit | 114 (34.76%) | 64 (29.36%) | 12 (25.00%) | 38 (61.29%) | |
| Intermediate-fit | 17 (5.18%) | 13 (5.96%) | 1 (2.08%) | 3 (4.84%) | |
| Frail | 73 (22.26%) | 65 (29.82%) | 5 (10.42%) | 3 (4.84%) | |
| Missing | 124 (37.80%) | 76 (34.86%) | 30 (62.50%) | 18 (29.03%) | |
| FISH | 0.044 | ||||
| Standard-risk | 203 (61.89%) | 140 (64.20%) | 30 (62.50%) | 33 (53.20%) | |
| High-risk | 62 (18.90%) | 36 (16.51%) | 8 (16.70%) | 18 (29.00%) | |
| Missing | 63 (19.21%) | 42 (19.27%) | 10 (20.80%) | 11 (17.70%) |
- Abbreviations: ALB, albumin; eGFR, estimated glomerular filtration rate; FISH, fluorescence in situ hybridization; GA score, geriatric vulnerability score; LDH, lactic dehydrogenase; β2-MG, beta 2 microglobulin.
3.2 Front-line induction regimens and efficacy
Of all the 328 patients, 75 patients who did not complete two cycles of treatment were not applicable for response evaluation. In total, 253 patients received a median 8 (2–12) cycles of induction chemotherapy. There as no differences among the three groups in terms of median front-line cycles (eight cycles in group 1 and 3, nine in group 2, p = 0.133). There were 185 patients whose response were PR or better and the total ORR was 73.12% (185/253). The ORRs of groups 1, 2, and 3 were 71.08%, 66.67%, and 85.42%, respectively. The proportion of patients in each group who achieved deep response (VGPR or better, ≥VGPR) was 39.16%, 25.64% and 62.50%, respectively. The ORR and deep response rates of group 3 were higher than group 1 and 2 (p = 0.016 and p = 0.018) (Table 3). Multivariable analysis controlling for age, GA score, eGFR, ALB, ISS and FISH risk confirmed an independent effect of the type of front-line inducion regimens administered in the odds of achieving VGPR or better. PIs + IMiDs regimens represented deeper response compared with PIs or IMiDs only regimen (adjusted OR, PIs + IMiDs vs. PIs = 0.33; 95% CI 0.13–0.96, p = 0.042; adjusted OR, PIs + IMiDs vs. IMiDs = 0.29; 95% CI 0.11–0.72, p = 0.043) (Table 4).
| Induction treatment effect | Total (n = 253) | PIs (Group 1, n = 166) | IMiDs (Group 2, n = 39) | PIs + IMiDs (Group 3, n = 48) | p value |
|---|---|---|---|---|---|
| ORR | 185 (73.12%) | 118 (71.08%) | 26 (66.67%) | 41 (85.42%) | 0.016 |
| CR + VGPR | 105 (41.50%) | 65 (39.16%) | 10 (25.64%) | 30 (62.50%) | 0.018 |
| PR | 80 (31.62%) | 53 (31.92%) | 16 (41.03%) | 11 (22.92%) | 0.069 |
| <PR | 68 (26.88%) | 48 (28.92%) | 13 (33.33%) | 7 (14.58%) | 0.013 |
- Abbreviations: CR, complete response; IMiDs, immune modulators; ORR, overall response rate; PIs, proteasome inhibitors; PR, partial response; VGPR, very good partial response.
| Adjusted OR | 95% CI | p value | |
|---|---|---|---|
| Front-line regimens | |||
| PIs + IMiDs | 1 | ||
| PIs | 0.33 | 0.13–0.96 | 0.042 |
| IMiDs | 0.29 | 0.11–0.72 | 0.043 |
| Age at diagnosis | |||
| 65–70 | 1 | ||
| 71–79 | 0.65 | 0.04–2.73 | 0.842 |
| ≥80 | 0.79 | 0.11–1.01 | 0.714 |
| GA score | |||
| Fit | 1 | ||
| Intermediate fit | 1.38 | 0.69–4.67 | 0.181 |
| Frail | 1.07 | 0.32–3.49 | 0.127 |
| ISS stage | |||
| I/II | 1 | ||
| III | 1.42 | 0.21–2.99 | 0.998 |
| eGFR | |||
| ≥40 ml/min | 1 | ||
| <40 ml/min | 1.30 | 0.42–2.37 | 0.638 |
| ALB | |||
| ≥30 g/L | 1 | ||
| <30 g/L | 1.01 | 0.47–1.64 | 0.073 |
| FISH | |||
| Standard-risk | 1 | ||
| High-risk | 0.98 | 0.16–2.37 | 0.60 |
- Abbreviations: ALB, serum albumin; eGFR, estimated glomerular filtration rate; FISH, fluorescence in situ hybridization; GA score, geriatric vulnerability score; ISS, International Scoring System.
3.3 Front-line maintenance treatment
Among 185 patients whose response to induction therapy was PR or better, 129 continued maintenance therapy, while the other 56 withdrew further treatment. The median duration of maintenance was 26 (IQR 16–30) months, including 92 patients receiving IMiDs (73 lenalidomide and 19 thalidomide), 20 with PIs (16 ixazomib and 4 bortezomib), and 17 with PI + IMiDs (ixazomib + lenalidomide). During the course of maintenance treatment, the most common reasons of drug discontinuation were hematologic toxicity, rash, and diarrhea, however it was difficult to determine the specific incidence rate due to less detailed records.
3.4 Long-term survival analysis
3.4.1 The results of the univariate and multivariate analysis
In this study, there were 21 patients missing follow-up and 307 patients were available for survival analysis. Of these, 66 died and 241 survived. The median PFS and OS of the entire cohort were 26 (IQR 12.00–42.8) months and 60 (IQR 40.00–67.20) months.
As shown in Table 5, univariate analysis revealed that age >70 years, GA score indicating frailness, R-ISS stage III, high LDH, hypercalcemia, induction therapy efficacy < PR, and no maintenance were unfavorable predictive factors of PFS (p < 0.05). Age >70 years, GA score of frailty, R-ISS stage III, high LDH, hypercalcemia, eGFR ≤40 ml/min, high-risk FISH, and no maintenance were risk factors predicting OS (p < 0.05). Yet, multivariate analysis revealed that only GA score of frailty, induction therapy effect < PR, and no maintenance were independent prognostic factors of poor PFS (p < 0.05), and age >70 years, GA score of frailty, initial response worse than PR, and no maintenance were independent prognostic factors predicting poor OS (p < 0.05).
| Risk factors for PFS | Univariate analysis | Multivariate analyses | ||
|---|---|---|---|---|
| HR | p | HR | p | |
| Age >70 year old | 1.379 (1.022–1.862) | 0.036 | 1.084 (0.696–1.688) | 0.722 |
| Hypercalcemia (≥2.75 mmol/L) | 1.916 (1.031–2.980) | 0.004 | 1.537 (0.841–2.808) | 0.162 |
| eGFR≤40 mL/min | 1.213 (0.846–1.741) | 0.294 | ||
| High LDH ≥250 IU/L | 1.671 (1.145–2.439) | 0.008 | 1.503 (0.746–3.027) | 0.254 |
| ISS stage III | 0.949 (0.693–1.299) | 0.743 | ||
| RISS stage III | 1.675 (1.200–2.339) | 0.002 | 0.986 (0.558–1.741) | 0.961 |
| FISH high-risk | 1.236 (0.856–1.785) | 0.258 | ||
| GA score frail | 1.906 (1.101–1.891) | 0.008 | 1.998 (1.284–3.110) | 0.002 |
| Induction efficacy < PR | 3.227 (2.125–4.900) | 0.001 | 2.423 (1.424–4.122) | 0.001 |
| No maintenance treatment | 1.961 (1.255–3.066) | 0.003 | 4.689 (3.226–10.032) | 0.001 |
| Risk factors for OS | Univariate analysis | Multivariate analyses | ||
|---|---|---|---|---|
| HR | p | HR | p | |
| Age >70 year old | 1.938 (1.313–2.861) | 0.001 | 2.303 (1.194–3.245) | 0.045 |
| Hypercalcemia (≥2.75 mmol/L) | 2.771 (1.653–4.643) | 0.001 | 2.113 (0/900–4.963) | 0.086 |
| eGFR ≤40 mL/min | 1.811 (1.184–2.769) | 0.006 | 1.026 (0.459–2.296) | 0.949 |
| High LDH ≥250 IU/L | 1.895 (1.187–3.024) | 0.007 | 2.198 (0.522–9.259) | 0.283 |
| ISS stage III | 1.023 (0.684–1.531) | 0.911 | ||
| RISS stage III | 2.109 (1.354–3.284) | 0.001 | 1.233 (0.811–2.788) | 0.554 |
| FISH high-risk | 1.598 (1.003–2.547) | 0.048 | 1.647 (0.450–6.033) | 0.451 |
| GA score frail | 2.173 (1.298–3.636) | 0.003 | 2.858 (1.434–5.694) | 0.003 |
| Induction efficacy < PR | 3.869 (2.339–6.400) | 0.001 | 3.087 (1.532–6.220) | 0.002 |
| No maintenance treatment | 2.305 (1.315–4.042) | 0.004 | 2.809 (1.506–5.376) | 0.001 |
- Note: Bold indicates values less than 0.05.
- Abbreviations: eGFR, estimated glomerular filtration rate; FISH, fluorescence in situ hybridization; GA score, geriatric vulnerability score; ISS, International Scoring System; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression-free survival; PR, partial response; R-ISS, Revised International Scoring System.
3.4.2 The effect of maintenance treatment on survivability
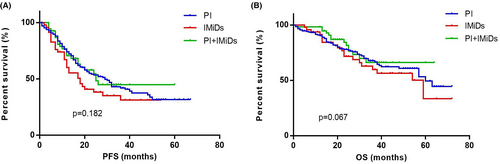
The PFS in group 1, 2, 3 were 28 (IQR 13.00–39.25) months, 18 (IQR 11.00–22.00) months and 26 (IQR 12.00–33.00) months respectively. There were no significant differences in PFS among the three groups (p = 0.182). The OS in group 1, 2, 3 was 60 (IQR 29.00–71.00) months, 59 (IQR 23.00–69.00) months and not reached (IQR 25.00–NR) respectively (p = 0.067) (Figure 1).
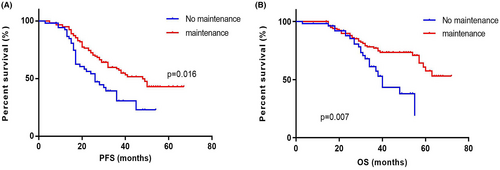
The PFS and OS of patients (n = 56) who did not receive maintenance therapy was 26 (IQR 16.00–36.00) months and 40 (IQR 30.00–49.00) months. The PFS and OS of patients (n = 129) who received maintenance therapy was 48 (IQR 23.00–63.00) months and not reached (IQR 38.00–NR). The PFS and OS of patients did not receive maintenance therapy were both significantly shorter than those who did (p = 0.016 and p = 0.007) (Figure 2).
3.4.3 The effect of age on survivability
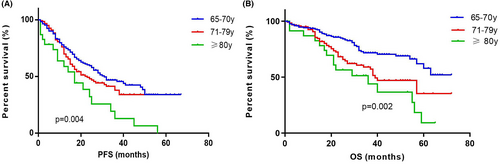
The PFS of patients aged 65–70 years (n = 166), 71–79 years (n = 117), and ≥80 years (n = 24) were 31 (IQR 14.00–45.20) months, 23 (IQR 12.00–40.00) months and 14 (IQR 7.00–24.00) months. The OS were not reached (IQR 32.00–NR), 39 (IQR 21.00–62.00) months and 37 (IQR 21.00–59.00) months. The PFS showed significant differences among different age groups (p = 0.004). The OS in age 65–70 group was significantly longer than those in age 71–79 and ≥80 groups (n = 0.002) but no differences were observed in OS between age 71 and 79 group and ≥80 group (p = 0.137) (Figure 3).
3.4.4 The effect of induction response on survivability
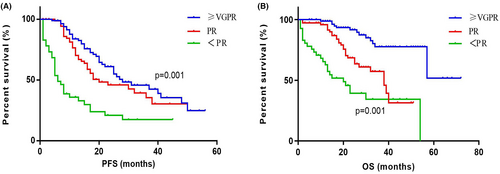
The PFS of patients with different responses (≥VGPR [n = 105], PR [n = 80], and <PR [n = 68]) were 28 (IQR 17.00–48.00) months, 20 (IQR 12.00–33.00) months, and 6 (IQR 4.00–22.00) months. The PFS showed significant differences among different response groups (p = 0.001). The corresponding OS of the three groups were not reached (IQR 57.00–NR), 38 (IQR 20.00–62.00) months, and 20 (IQR 8.00–54.00) months. The OS showed significant differences among different response groups (p = 0.001) (Figure 4).
3.4.5 The effect of GA score on survivability
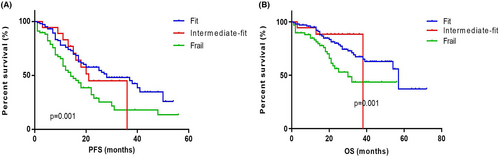
The patients with GA scores (n = 204) were classified into fit (0 score, n = 114, 55.88%), intermediate fit (1 score, n = 17, 8.33%), and frail (≥2 score, n = 73, 35.78%). The PFS were 28 (IQR 13.00–39.50) months, 21 (IQR 15.00–36.00) months and 14 (IQR 8.00–30.00) months. The OS were 57 (IQR 27.00–59.00) months, 38 (IQR 36.00–39.00) months and 30 (IQR 18.00–49.00) months. The PFS and the OS of the frail and intermediate fit groups were both significantly shorter than that of fit group (p = 0.001 and p = 0.001) (Figure 5).
4 DISCUSSION
In Chinese current clinical practice, MM patients aged ≥65 years are defined as elderly and considered ineligible for transplantation. Herein, we analyzed the treatment patterns of elderly MM patients from four big myeloma centers in China over the last 5 years. Our study suggests that even novel therapies including PIs and IMiDS are commonly administrated, age >70, general performance frailty, induction response < PR and no maintenance treatment are independent risk factors for OS.
Our data demonstrated distribution of front-line regimens in elderly MM patients in multiple centers of China. Due to quick approval and reimbursement of medical insurance during past 5 years, PIs and IMiDs based regimens were prefered in front-line treatment. In this study no patient received anti-CD38 daratumumab because it was not approved as front-line therapy in China until 2021.
Current treatment options for transplant-ineligible MM patients are not unique, and clinical trials have shown that regimens containing two kinds of new drugs can further improve ORR and PFS compared with regimens containing only one kind of new drug.13-15 In these clinical tirals, the addition of anti-CD38 daratumumab on the basis of bortezomib, melphalan and prednisone (Dara-VMP) or lenalidomide and dexamethasone (Dara-Rd) resulted in ORR over 90% and even longer PFS and OS. In our study we found that in real world PIs combined with IMiDs regimens produced the comparable ORR but the OS was not as good as clinical trials. In this study there were no differences in OS among patients treated with different first-line induction regimen, which is different from previous real-world reports.5, 6 We supposed it may be related to the regimen heterogeneity in different studies. Considering the clinical benefit of Dara-Rd in transplant-ineligible MM patients regardless of frailty status in clinical trial, it is worthy to explore the combination of daratumumab to the PIs or IMiDs regimens in elderly patients for further survival improvement in real world .
Maintenance treatment had a particular impact on the survival of elderly MM patients as response was further deepened.16 Our real world study also confirmed that the maintenance treatment after obtaining the response prolonged the PFS and OS in elderly MM patients. It is worth noting that about 30% (56/185) of the responders did not start first-line maintenance treatment at the end of induction therapy. The original record did not tell us why these 56 patients refuse maintenance. But these data showed that elderly MM patients are not adequately treated in China. There were urgent need to improve this aspect.
In this study we found a significant correlation between first-line induction response depth and survival. Similarly, the SWOGS0777 trial showed the deeper response of induction treatment represented the longer PFS and OS.13 Also the FIRST trial analysis showed 4-year survival rates was closely related to the depth of induction response.16 Different from the above, a meta-analysis suggested achieving CR was an independent predictor of long-term outcome regardless of age and ISS stage.17 Anyhow in the new drug era, to pursue deep response in elderly patients is possible and still valuable. It is not only the induction regimens but the front-line response that matters.
For patients with MM, various studies suggest that age is an important prognostic factor. In the era of traditional chemotherapy, the median OS in elderly MM population including 60–69, 70–79, and ≥80 years groups were only 20, 12, and 6 months, respectively.18 After novel agents were widely used, OS in corresponding age groups were remarkably prolonged in the real practice such as 22 months in patients over 80 years.5 This current study also showed that the older age means worse survival, but new drugs has significantly improved the outcome of elderly patients in different age groups especially the OS in ≥80 years group reached 37 months.
Due to comorbidities as well as the poor physical performance, a considerable part of elderly patients were excluded from clinical trials. Actually the assessment of frailty is very important because this can affect not only treatment choices but also clinical outcomes. In clinical trials, the GA score was found to be an independent predictor of OS, regardless of the patients' ISS stage, cytogenetic abnormalities, or treatment regimen.10 The European Myeloma Network proposed that elderly MM patients should adopt different treatment intensities according to their fitness,19 but currently the GA score is not widely used in the real world. The proportion of frail patients in our study was 35.78% (73/204) which was close to the report by Li et al.20 Our data suggested that GA score indicating fitness was independently correlated with longer OS, which was consistent with the conclusions in clinical trial.10 In a retrospective study all the patients were treated with bortezomib-based regimen and the frail patients had shorter OS compared with fit and moderate-fit patients (49 months vs. 63 months vs.63 months, p < 0.05).21 Together, these data verify the prognostic value of GA score in real practice.
In this study, factors such as high-risk cytogenetic abnormalities and R-ISS stage III, which were assumed to predict poor OS, did not show independent prognostic value. A recent report on clinical trial data indicated that the relative contribution of different prognostic factors in predicting disease risk varies according to age, and for older MM patients, frailty score and comorbidity assessment are more important than FISH and ISS stage.22 Considering that the prognostic value of these factors was mainly established through clinical trials, their value in the real world may require further research.
This study had some limitations. First, this was a retrospective study with unmatched clinical characteristics at baseline in different treatment regimens. But after we adjusted the confounding factors, PIs + IMiDs combination regimens still showed superior response. Second, adverse events and toxicity were not recorded detailed and absence of this aspect was a pity.
In summary, our cohort represented the real-world treatment situations in elderly MM patients in China. Induction regimens containing PIs + IMiDs produced deeper overall response rate than only PIs or IMiDs based regimens and should be recommended to suitable patients. The maintenance treatment can further improve the outcome and this aspect needs our special attention.
AUTHOR CONTRIBUTIONS
Li Bao: Writing – original draft (equal). Ai-Jun Liu: Writing – original draft (equal). Bin Chu: Resources (equal). Qian Wang: Resources (equal). Yu-Jun Dong: Resources (equal). Min-Qiu Lu: Resources (equal). Lei Shi: Resources (equal). Shan Gao: Resources (equal). Yu-Tong Wang: Resources (equal). Li-Fang Wang: Formal analysis (equal). Weng-Ming Chen: Resources (equal); supervision (equal). Jun-Ling Zhuang: Conceptualization (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
We would like to thank the faculty members who collected samples and assembled the data.
CONFLICT OF INTEREST
The authors have stated that they have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The processed data required to reproduce these findings cannot be shared at this time as the data also is a part of an ongoing study.




