Influence of the skeletal muscle index on pharmacokinetics and toxicity of fluorouracil
Eduard Schmulenson and Nigina Zimmermann contributed equally to this work (listed in alphabetical order).
Abstract
Background
The body composition of patients has been associated with tolerability and effectiveness of anticancer therapy. This study aimed to assess the influence of the skeletal muscle index (SMI) on the pharmacokinetics and toxicity of fluorouracil.
Methods
Patients treated in an oncological practice with fluorouracil-based chemotherapy and undergoing therapeutic drug monitoring were retrospectively investigated. Computed tomography images were analyzed to measure abdominal skeletal muscle areas in Hounsfield units for the psoas major muscle, back and total skeletal muscle to determine the SMI. For the latter, an automated segmentation method was used additionally. SMI measures were tested as covariates on fluorouracil clearance in a population pharmacokinetic model. Furthermore, regression analyses were performed to analyze the influence of SMI measures on the probability of clinically relevant adverse events (CTCAE grades ≥ 2).
Results
Fluorouracil plasma concentrations of 111 patients were available. Covariate analyses showed significant improvements of the model fit by all SMI measures. However, interindividual variability of fluorouracil clearance was only slightly reduced, whereas the SMI of the back muscle showed the largest reduction (−1.1 percentage points). Lower SMI values of the back muscle increased the probability for polyneuropathy and lower SMI of the psoas increased the probability for fatigue.
Conclusions
Our results suggest that pharmacokinetics and toxicity of fluorouracil may be associated with specific SMI measures which deserve further investigation.
1 INTRODUCTION
Fluorouracil (5FU) is still one of the cornerstones for the treatment of various solid tumors, particularly colorectal and head and neck cancer.1-3 Typically, 5FU is dosed according to the patient's body surface area (BSA), resulting in a wide range of variability in 5FU plasma concentrations.4 This pharmacokinetic variability may result individually in an insufficient response to 5FU therapy or intolerable toxicity, leading to treatment discontinuations. In fact, approximately 60% of patients treated with 5FU are reported being underdosed, whereas about 15% being overdosed when BSA-based dosing is applied.5
Due to its hydrophilic nature, the volume of distribution of 5FU is highly correlated with lean body mass (LBM) which includes muscle mass.6 Since BSA does not account for changes in body composition, this additional knowledge could be of high interest when sarcopenic cancer patients are treated with anticancer drugs.7 Several studies found that sarcopenia in cancer patients is a predictor for low overall survival in various tumors.8-12 An association between muscle status and toxicity under therapy with various anticancer drugs was shown as well.12 In particular, patients with a dose-limiting toxicity under 5FU chemotherapy had a higher 5FU dose per kg of LBM compared to patients with a lower grade toxicity.13, 14 Other metrics of body composition (total body water, fat-free mass) could be linked to 5FU pharmacokinetics,15 whereas a LBM-normalized 5FU dose was not correlated with 5FU exposure, defined as area under the concentration-time curve (AUC).16
Sarcopenia is usually defined by a reduction in skeletal muscle index (SMI) which is calculated by the total muscle cross-sectional area at the third lumbar vertebra (L3) normalized to the squared patient's height (cm2/m2).17 So far, the relationship of SMI with 5FU pharmacokinetics has not been evaluated. The aim of this study was therefore to investigate the influence of the SMI on 5FU pharmacokinetics as well as 5FU-associated toxicity.
2 METHODS
2.1 Patients and data
In this study, patients under a 5FU-based, infusional chemotherapy from the oncological outpatient clinic UnterEms in Leer, Germany, were retrospectively analyzed. Patients with documented therapeutic drug monitoring of 5FU, that is, quantification of 5FU plasma concentrations, and at least one computed tomography (CT) scan of the L3 area were included for analysis. 5FU plasma concentrations were obtained at steady state during continuous infusion and quantified using the My5-FU™ immunoassay (Saladax Biomedical Inc., Bethlehem, PA, USA) with a lower limit of quantification of 86 ng/ml.18 Dose adjustments were performed at the discretion of the treating oncologist. Adverse events (AE) were graded at each patient visit according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.19 In order to ensure that the muscle status corresponded to measured 5FU plasma concentrations and AE, a maximum time frame between CT scan and blood sampling/AE documentation had to be defined. Chung et al. found a median change in SMI values of 8.7% in patients with stage III or high-risk stage II colon cancer treated with the FOLFOX scheme (5FU, folinate, oxaliplatin) within 210 days between preoperative and post-chemotherapy CT.20 In addition, the mean measurement error of SMI quantification via CT scans was reported to be 8.5%.21 Based on this information, a maximum time frame of ±205 days between CT scan and blood sampling was defined excluding patients with a larger temporal distance between SMI and plasma concentration measurements/AE documentations from the analysis. The study was approved by the ethics committee at the Faculty of Medicine of the University of Bonn (protocol code 014/18).
2.2 Image analysis
The SMI was assessed using routinely collected CT images from different radiological practices. As recommended by the European Working Group on Sarcopenia in Older People, measurements were performed at the L3 level since the individual skeletal muscle areas correlated the most with overall skeletal muscle.22 Muscle areas were measured based on Hounsfield units (HU) as well as an automated segmentation method provided by the software sliceOmatic® version 5.0 (TomoVision, Magog, Canada).23 The HU range was set to the density of skeletal muscle (35–50 HU) to exclude any areas of different tissues.24 Using this “Hounsfield method,” the psoas major, back muscle, and total skeletal muscle at the L3 level were examined. For the latter, the automated segmentation method was additionally used. An overview of the different SMI measures and the respective muscle areas is provided in the Supporting Information (SI) 1, Table S1-1. All SMI measures were obtained by dividing the respective measurements by the squared patient's height.
2.3 Population pharmacokinetic analysis
The influence of the different SMI measures on 5FU pharmacokinetics (PK) was analyzed in a population PK model of 5FU. This model was initially developed at the Department of Clinical Pharmacy at the University of Bonn using data from the study of Wilhelm et al.25 Modeling was performed with the non-linear mixed effect modeling software NONMEM® version 7.226 combined with implemented scripts in PsN (version 3.6.2).27, 28 NONMEM® uses the maximum likelihood method to simultaneously estimate population values of fixed-effect parameters (e.g., drug clearance) and values of random-effect variables (e.g., interindividual and residual variability) in order to obtain individual parameters. Model parameters were estimated by the first-order conditional estimation method with interaction.26 The likelihood-ratio test was used to discriminate between nested models. A nested model was considered superior to another when the objective function value (OFV), provided by NONMEM®, was reduced by 3.84 points (chi-square value, p < 0.05, one degree of freedom). Covariates which are able to explain interindividual variability (IIV) in 5FU clearance and volume of distribution were investigated as well, including age, sex, infusion time (24 or 46 h, coded as a binary covariate), laboratory parameters (creatinine, bilirubin, ALT, AST, GGT, LDH), tumor markers (CA 19–9, CEA), and BSA. These were implemented into the model in a stepwise forward inclusion and backward elimination approach using the scm script provided by PsN with an included fixed set of parameter-covariate parametrizations (linear, piece-wise linear, exponential, power relations).27, 28 In the forward inclusion step, covariates which led to a significant decrease of the OFV (p < 0.05) were kept for further evaluation. This model was then re-evaluated by backward elimination of each included covariate with a significance level of p < 0.01. If a covariate was still significant in this step, it was eventually kept in the model. Model robustness and precision and bias of parameter estimates were evaluated by a non-parametric bootstrap analysis without stratification. Median and 95% confidence intervals of parameter estimates were derived from 1000 replicate datasets obtained from sampling individuals from the original dataset with replacement.
The population pharmacokinetic model was then applied to the dataset of this study and revised, where necessary. In this population PK analysis, NONMEM® version 7.529 and PsN (version 5.0.0)27, 28 were used for model development and R (version 4.1.0)30 was used for visualization of results. Piraña (version 2.9.7) served as front interface.31 Based on this revised model, a covariate analysis was performed in order to explore if the different SMI measures had an influence on 5FU clearance and volume of distribution. Each SMI measure was individually tested as a covariate on 5FU PK parameters and included if a statistically significant reduction (p < 0.05) of the OFV was found. Exponential functions were tested to describe the relationship between the different SMI measures and 5FU PK parameters. The model fit was assessed by goodness-of-fit plots32 and prediction-corrected visual predictive checks33 based on 1000 dataset simulations. Additionally, a non-parametric bootstrap analysis without stratification, as described above, was performed.
2.4 Logistic regression
3 RESULTS
3.1 Patient characteristics
The dataset consisted of routinely collected data from 175 patients between September 2014 and July 2020. Twenty of them had to be excluded due to missing CT images. Further 44 patients were excluded because the time frame between their CT scan and blood sampling was longer than 205 days (see “Methods” section). The remaining 111 patients were included for further analyses (Table 1). For the development of the population PK model, 395 5FU plasma concentration measurements were included. All included patients received 24-h infusions of 5FU.
| Characteristic | Median (range) or n |
|---|---|
| Demographics | |
| Sex | |
| Male | 75 |
| Female | 36 |
| Age (years) | 64 (35–84) |
| Body surface area (m2) | 1.97 (1.47–2.85) |
| Skeletal muscle indices (SMI) | |
| SMI psoas major (cm2/m2) | 1.48 (0.46–3.78) |
| SMI back muscle (cm2/m2) | 3.78 (0.94–8.41) |
| SMI total muscle (Hounsfield method) (cm2/m2) | 9.58 (4.12–18.82) |
| SMI total muscle (Segmentation method) (cm2/m2) | 50.26 (25.47–92.67) |
| Therapy-related details | |
| 5FU dose (mg/m2) | 2283 (1441–3641) |
| 5FU AUCa (mg × h/L) | 19.7 (2.1–45.0) |
| Number of observed cycles per patient | 2 (1–5) |
| Therapy regimen | |
| AIOb | 26 |
| FUFOXc (including monoclonal antibodies) | 22 |
| Paclitaxel/cisplatin/5FU/folinate | 30 |
| Other | 32 |
| Tumor entity | |
| Colorectal cancer | 56 |
| Gastroesophageal cancer | 33 |
| Pancreatic cancer | 12 |
| Other | 10 |
- a Calculated by multiplying the infusion time with the measured steady-state concentration.
- b Weekly 5FU infusion (2600 mg/m2) over 24 h in combination with folinate (500 mg/m2).
- c Weekly 5FU infusion (2000 mg/m2) over 24 h in combination with folinate (500 mg/m2) and oxaliplatin (50 mg/m2).
3.2 Influence of the skeletal muscle index on 5FU pharmacokinetics
First, an initial population PK model based on data from the study of Wilhelm et al.25 was developed. A one-compartment model with linear elimination turned out to be the best model to describe 5FU disposition. IIV terms were implemented on 5FU clearance, volume of distribution, and residual variability. The latter consisted of an additive as well as a proportional term.34 However, due to model instabilities, estimates of the volume of distribution and its IIV had to be fixed to previously estimated values. Based on analyses of rich PK data from our group and data from the Cantonal Hospital St. Gallen, Switzerland, volume of distribution and its IIV were fixed to 46.1 L and 51.1%, respectively.35-37 Therefore, covariates could only be tested on 5FU clearance. After the forward inclusion step of the covariate analysis, BSA, infusion time, and LDH concentration were found to be significant linear covariates on 5FU clearance. LDH concentration was excluded after performing the backward elimination step. Ultimately, BSA and infusion time remained in the model. However, the bootstrap analysis revealed the estimate of the infusion time effect to be unreliable since its 95% confidence intervals included zero. Hence, this parameter was excluded from the final model. In Table 2, the development steps of the population PK model are shown. Table 3 depicts the final population PK parameter estimates as well as the median values of the bootstrap analysis.
| Model number | Description | OFV | ∆OFV | p value |
|---|---|---|---|---|
| 1 |
Base model
|
−611.310 | 0 | — |
| 2 |
Covariate model after forward inclusion step
|
−653.107 | −41.797 | <0.0001a |
| 3 |
Covariate model after backward elimination step
|
−647.132 | +5.975 | 0.0145 |
| 4 |
Final model
|
−637.090 | +10.042 | 0.0064 |
- Abbreviations: ∆OFV, Difference in objective function value; BSA, Body surface area; IIV, Interindividual variability; OFV, Objective function value.
- a Three degrees of freedom.
| Parameter | Estimate | Bootstrap median (95% confidence intervals) |
|---|---|---|
| CL5FU [L/h] | 209 | 209 (198–220) |
| V5FU [L] | 46.1 (fixed) | 46.1 (fixed) |
| BSA effect on CL5FU | 0.681 | 0.688 (0.455–0.933) |
| Interindividual variability | ||
| CL5FU [%CV] | 16.3 | 15.5 (10.3–19.2) |
| V5FU [%CV] | 51.1 (fixed) | 51.1 (fixed) |
| Residual variability [%CV] | 37.5 | 36.1 (18.7–52.2) |
| Residual variability | ||
| Additional error term [ng/ml] | 77.4 | 87.2 (54.7–139) |
| Proportional error term [%] | 19.8 | 18.8 (14.7–23.6) |
- Abbreviations: BSA, body surface area; CL, clearance; CV, coefficient of variation; V, volume of distribution.
The developed population PK model was applied to the dataset of this study. Running this initial model revealed that the estimate of the additive term of the residual variability ran into a boundary close to zero. In addition, the IIV term on residual variability was estimated with a relative standard error (RSE) of 86% and shrinkage38 of 76%. Both parameters were assumed to be negligible and thus removed from the model leading to a non-significant increase in OFV (Table 4).
| Model number | Description | OFV | ∆OFV | p value | IIV Clearance [%CV] |
|---|---|---|---|---|---|
| 1 | Initial model without BSA as covariate (Table 3) | −754.341 | 0 | — | 26.6 |
| 2 | Initial model with BSA as covariate (Table 3) | −792.218 | −37.877 | <0.00001 | 22.0 |
| 3 | Initial model with BSA as covariate (Table 3), without additive term and IIV term of residual variability (Revised model) | −791.624 | +0.594 | (0.74a, referring to model 2) | 22.0 |
| 3a | Revised model + SMI total muscle (Hounsfield method) | −798.302 | −6.678 | 0.0098 | 21.5 |
| 3b | Revised model + SMI total muscle (segmentation method) | −798.408 | −6.784 | 0.0092 | 21.1 |
| 3c | Revised model + SMI psoas major | −799.070 | −7.446 | 0.0064 | 21.1 |
| 3d | Revised model + SMI back muscle | −803.910 | −12.286 | 0.00046 | 20.9 |
- Abbreviations: ∆OFV, Difference in objective function value; BSA, Body surface area; CV, Coefficient of variation; IIV, Interindividual variability; OFV, Objective function value; SMI, Skeletal muscle index.
- a Two degrees of freedom.
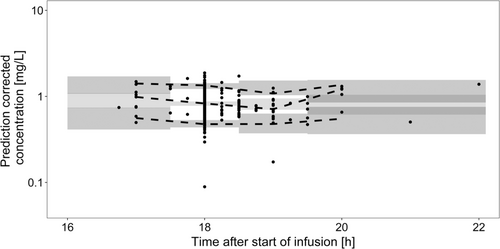
The prediction-corrected visual predictive check (Figure 1) as well as goodness-of-fit plots (see SI 2, Figure S2-1) of the final model showed a reasonable model fit. Final parameter estimates along with bootstrap results are presented in Table 5. The NONMEM® code of the final model is outlined in SI 3.
| Parameter | Estimate (relative standard error, %) | Shrinkage [%] | Bootstrap median (95% confidence intervals) |
|---|---|---|---|
| CL5FU [L/h] | 223 (2.4) | 223 (212–234) | |
| V5FU [L] | 46.1 (fixed estimate) | 46.1 (fixed estimate) | |
| BSA effect on CL5FU | 0.794 (14.5) | 0.796 (0.543–1.02) | |
| SMIBack effect on CL5FU | 0.0570 (29.8) | 0.0575 (0.0283–0.0885) | |
| Interindividual variability | |||
| CL5FU [%CV] | 20.9 (7.4) | 15.1 | 20.4 (15.8–24.5) |
| V5FU [%CV] | 51.1 (fixed estimate) | 100 | 51.1 (fixed estimate) |
| Residual variability | |||
| Proportional error [%] | 21.4 (3.3) | 10.6 | 21.4 (19.0–23.8) |
- Abbreviations: BSA, body surface area; CL, clearance; CV, coefficient of variation; SMIback, skeletal back muscle index; V, volume of distribution.
3.3 Influence of skeletal muscle indices on toxicity under 5FU therapy
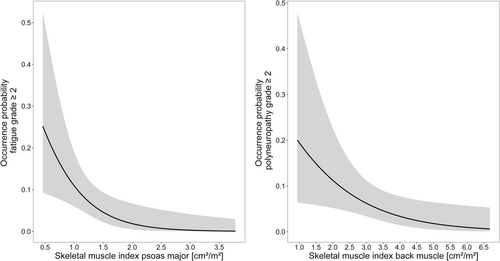
For every AE, the number of patients suffering from AE grade 2 or higher is presented in SI 4, Table S4-1. The logistic regression analysis showed statistically significant correlations for two AE. The SMI of the psoas major was significantly correlated to the fatigue syndrome as well as the SMI of the back muscle to the occurrence of clinically relevant polyneuropathy. An increase of the respective SMI by 1 cm2/m2 decreased the probability of developing the identified AE ≥ grade 2 by 85% and 48%, respectively. The final logistic regression analyses are depicted in Figure 2. Presentations of the odds ratios for all investigated AE are outlined in SI 4, Table S4-2.
4 DISCUSSION
This is the first study evaluating the influence of different SMI measures on 5FU PK and toxicity. The results suggest that selected SMI measures may be associated with 5FU PK. Interpreting the individual SMI measures, it should be kept in mind that SMI parameter values which were obtained by the Hounsfield method were quantified using a range of 35–50 HU. This range differed from HU ranges of other studies13, 14, 39 and resulted in lower absolute values when, for example, comparing the SMI of the total muscle obtained by the Hounsfield method to the same SMI quantified by the segmentation method. However, the observed trends and associations were the same for all SMI measures analyzed in this study. Provided that these findings are confirmed in future prospective studies, the question remains how these findings may be transposed in real-world patients. In clinical routine, CT imaging of cancer patients is frequently performed, for example, for tumor staging. Therefore, even though muscle status assessment by CT imaging is not routinely conducted, there would already be raw data available to quantify different SMI measures.40
Our population PK model was able to adequately describe the observed routine data. However, the sole availability of steady-state concentrations impeded model development. The estimate of 5FU volume of distribution had to be therefore fixed and could not be used for covariate analysis. Gusella et al. found a significant relationship between total body water and 5FU volume of distribution as well as fat-free mass and 5FU volume of distribution.15 The initially developed 5FU population PK model, which was based on data from Wilhelm et al.,25 included BSA as the only significant covariate on 5FU clearance. In general, the identified covariates on 5FU PK vary considerably. A similar influence of BSA was identified in two other published population PK models as well,41, 42 whereas other models revealed significant effects of sex,36, 43 age,44 or body weight.45 When applying the initial model on the new dataset, all SMI measures were positively associated with 5FU clearance when adding them as covariates and significantly improved the model fit. The inclusion of the SMI of the back muscle led to the largest improvement which deserves further investigation on physiological plausibility. While this finding gives additional hints that body composition may influence the PK of 5FU and other anticancer drugs,15, 46-48 the minor reduction of IIV of 5FU clearance revealed that the usage of SMI for dose adjustment purposes may be of limited value. In fact, the inclusion of BSA had a higher impact on 5FU clearance (Table 4). The wide range of SMI measures (Table 1) may further explain the limited reduction of variability in clearance. Interestingly, Molenaar-Kuijsten et al. could not identify any relationships between the SMI-derived skeletal muscle mass and the 5FU elimination rate constant in 151 patients treated with its oral prodrug capecitabine.39 Williams et al. investigated the influence of LBM which was derived from the SMI on 5FU AUC but could not find any significant differences between sarcopenic and non-sarcopenic patients.16 However, they investigated a smaller patient cohort (25 patients) and only used first cycle plasma concentrations of 5FU, whereas in our study, plasma concentrations from different cycles were available.
As a limitation of our study, it should be noted that the genotypes and the activity of the main metabolizing enzyme, dihydropyrimidine dehydrogenase (DPD), which are important predictors for 5FU exposure49, 50 were not available for our patient cohort. However, IIV of 5FU clearance was already lower than the average IIV of 40% as reported in an extensive review on 5FU therapeutic drug monitoring by Beumer et al.5 This could be explained by the comparatively low prevalence of DPD risk variants which are associated with alleles producing DPD with minimal/no activity (DPYD*2A or DPYD*13) or with alleles producing DPD with decreased metabolic function (c.2846A > T or c.1129–5923C > G). Up to 1% of the European population are carriers of the non-active variants, whereas the two latter variants are present in about 5% of Europeans.51 Keeping in mind the number of patients used for model development (n = 75 in the study of Wilhelm et al.,25 n = 111 in the present study), only few patients presumably were carriers of these DPD risk variants that are associated with a substantial influence on the variability in 5FU clearance.
The findings of the logistic regression analysis showed several significant associations between specific SMI measures and adverse events under 5FU therapy which was generally in accordance with previous studies.13, 14, 16 Williams et al. found a significant positive association of LBM and oxaliplatin clearance and LBM and volume of distribution as well as a significant association of low LBM and high-grade toxicity in older patients with colorectal cancer. Similar results were identified for cisplatin even though dose-limiting toxicity was not significantly correlated with body composition.53 Our finding of a higher probability of the occurrence of clinically relevant polyneuropathy with decreasing SMI of the back muscle deserves further investigation. Since the psoas muscle is necessary for everyday movement, the reduction of its SMI may be an explanation for the identified increase in the probability of clinically relevant fatigue. It should be noted, however, that our analysis was focused on evaluating the general susceptibility of experiencing clinically relevant AE depending on body composition in patients treated with a 5FU-based chemotherapy. Regarding concomitant chemotherapy, only qualitative information of its administration was available for our study. In a future study, it would be of high interest to investigate the influence of individual drug PK on AE development in order to distinguish between the respective individual contributions of these drugs. In addition, the patient's performance status should be included in such an analysis as it was reported to be significantly associated with SMI54 and a significant predictor for drug toxicity under 5FU-based chemotherapy.55
In conclusion, this retrospective study gives first hints that the SMI as a measure of body composition may be associated with the pharmacokinetic disposition and the development of toxicity of 5FU. A prospective study in which SMI measures and 5FU pharmacokinetics are investigated should provide additional insights into these relevant relationships between body composition and clinical outcome of 5FU-based chemotherapy.
AUTHOR CONTRIBUTION
Designed research: ES, NZ, LM, UJ. Performed research: ES, NZ, LM. Analyzed data: ES, NZ, SK, IS, UJ. Wrote or contributed to the writing of the manuscript: ES, NZ, LM, SK, IS, UJ.
FUNDING INFORMATION
This study was not funded.
ACKNOWLEDGEMENT
Open Access funding enabled and organized by Projekt DEAL. WOA Institution: RHEINISCHE FRIEDRICH-WILHELMS-UNIVERSITAET BONN Consortia Name : Projekt DEAL
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest that are relevant to the content of this manuscript.
ETHICS STATEMENT
This retrospective study was approved by the local medical ethics committee.
CLINICAL TRIAL REGISTRATION
Due to the retrospective nature of this study, trial registration was not conducted.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon reasonable request.




