Antitumor effects of duvelisib on Epstein–Barr virus-associated lymphoma cells
Abstract
Epstein–Barr virus (EBV) is a ubiquitous oncogenic virus that is associated with B cell lymphomas, including Burkitt lymphoma and Hodgkin lymphoma. Previous studies have shown that the phosphatidylinositol 3-kinase (PI3K)/Akt pathway is activated in EBV-associated lymphomas and can be a novel therapeutic target. An oral dual inhibitor of PI3Kγ and PI3Kδ, duvelisib, is in clinical trials for the treatment of lymphoid malignancies. In this study, we evaluated how duvelisib affects the activity of the PI3K/Akt signaling pathway and if it has antitumor effects in EBV-associated lymphoma cell lines. We found that the PI3K/Akt signaling pathway was activated in most of the B and T cell lymphoma cell lines tested. Additionally, duvelisib treatment inhibited cellular growth in the tested cell lines. Overall, B cell lines were more susceptible to duvelisib than T and NK cell lines in vitro regardless of EBV infection. However, the additional influence of duvelisib on the tumor microenvironment was not assessed. Duvelisib treatment induced both apoptosis and cell cycle arrest in EBV-positive and -negative B cell lines, but not in T cell lines. Furthermore, duvelisib treatment reduced the expression of EBV lytic genes (BZLF1 and gp350/220) in EBV-positive B cell lines, suggesting that duvelisib suppresses the lytic cycle of EBV induced by B cell receptor signaling. However, duvelisib did not induce a remarkable change in the expression of EBV latent genes. These results may indicate that there is therapeutic potential for duvelisib administration in the treatment of EBV-associated B cell lymphomas and other B cell malignancies.
Introduction
Epstein–Barr virus (EBV) is the primary agent causing infectious mononucleosis and persists asymptomatically for life in nearly all adults. EBV infection is associated with the development of B cell lymphomas, posttransplant lymphoproliferative disease (PTLD), natural killer (NK)/T cell lymphoma, and chronic active EBV disease 1-4. Generally, EBV-PTLD disease results in a type III latency pattern characterized by the expression of all EBV latency-associated proteins. These include the EBV nuclear antigens (EBNA) 1, 2, 3A, 3B, and 3C (EBNA-1, EBNA-2, EBNA-3A, -3B, and -3C, respectively) and latent membrane proteins (LMP) 1 and 2 (LMP1, LMP2, respectively). Conversely, EBV-positive Burkitt lymphoma is usually characterized by a type I latency pattern and primarily expresses EBNA-1 1. Patients with EBV-associated lymphoid malignancies treated with cytotoxic chemotherapy are often refractory to it. Therefore, understanding the underlying molecular pathways of EBV-associated lymphoid malignancies is necessary to develop more effective treatment strategies.
LMP1 is considered a major EBV oncoprotein that mediates activation of multiple cellular signaling pathways, such as c-Jun-N terminal kinases (JNK), tumor necrosis factor (TNF) receptor associated factor TRAF, and nuclear factor kappa beta (NF-κB) signaling 5, 6. In 2003, Dawson et al. demonstrated that LMP1 can also activate phosphatidylinositol 3-kinase (PI3K) and induce subsequent activation of serine/threonine protein kinase (Akt) to promote cell survival 7. Subsequently, several other studies have shown that constitutive activation of the PI3K/Akt pathway via LMP1 is a key element of LMP1-mediated transformation. This suggests that the PI3K/Akt pathway can be a therapeutic target for the treatment of EBV-associated lymphoid malignancies 8-11. Furthermore, a recent study using genome-wide CRISPR/Cas9 screens in EBV-transformed B cells has revealed that the EBV oncoprotein LMP2A also activates the PI3K/Akt pathway 12.
The PI3K/Akt signaling pathway has been associated with many virus-associated cancers. Moreover, it is known to regulate numerous biological activities, including cellular growth, survival, and proliferation 13, 14. The PI3Ks are divided into three classes: I, II, and III. Of the three classes of PI3K isoforms, class I PI3K comprises four different catalytic isoforms including PI3Kα, β, γ, and δ 15. While PI3Kα and β are ubiquitously expressed in all mammalian tissues, PI3Kγ and PI3Kδ have more selective roles. PI3Kγ has a particular role in T cell activation and PI3Kδ expression is largely restricted to hematopoietic cells, and has a crucial role in mediating B-cell receptor (BCR) signaling, proliferation/survival, and migration 16. Furthermore, PI3Kδ is aberrantly activated in a variety of B cell malignancies such as chronic lymphocytic leukemia (CLL) and acute myeloid leukemia 17, 18. Therefore, PI3Kδ has emerged as a promising therapeutic target in hematological malignancies. In fact, in a phase III clinical trial testing the oral selective PI3Kδ inhibitor idelalisib in combination with rituximab, the survival of CLL patients improved considerably 19.
Duvelisib is a molecule previously found to inhibit the PI3K/Akt signaling pathway, thereby inhibiting BCR signaling, diminishing chemotaxis, and inhibiting cytokine-induced CLL cell proliferation with minimal apoptosis 20, 21. The duvelisib molecule closely resembles the chemical structure of idelalisib, but biochemically targets PI3Kγ in addition to PI3Kδ 22. It is expected that dual inhibition of PI3Kγ and PI3Kδ by duvelisib may be another therapeutic target for the treatment of CLL and may overcome resistance formed against idelalisib 23. Furthermore, clinical studies of duvelisib in indolent non-Hodgkin lymphoma and CLL have shown clinical activity 20, 24. However, the effects of PI3Kγ or PI3Kδ inhibitors on EBV-associated lymphoma cells have not been investigated. In this study, we evaluated the activity of the PI3K/Akt signaling pathway and antitumor effects of duvelisib on EBV-associated lymphoma cell lines.
Materials and Methods
Cell lines and reagents
The cell lines used in this study are summarized in Table 1. Lymphoblastoid cell line (LCL) was generated by infection of B cells with EBV (B95-8 strain). Akata (+) 25, Mutu I 26, Raji 27, and P3HR1 28 are EBV-positive B cell lines, and BJAB 29 and Akata (-) 30 are EBV-negative B cell lines. SNT16 31 is an EBV-positive T cell line, and Jurkat 32 and MOLT4 33 are EBV-negative T cell lines. KAI3 34 is an EBV-positive, and KHYG1 35 is an EBV-negative NK cell line. Duvelisib was obtained from Infinity Pharmaceuticals (Cambridge, MA) and was dissolved in DMSO. Idelalisib was purchased from Tokyo Chemical Industry (Tokyo, Japan) and was dissolved in DMSO.
| Cell type | Cell line | EBV (latency pattern) | Cell origin |
|---|---|---|---|
| B cell lines | BJAB | - | Burkitt lymphoma |
| Akata (-) | - | EBV-negative clones from Akata | |
| Akata (+) | + (I) | EBV-related Burkitt lymphoma | |
| Mutu I | + (I) | EBV-related Burkitt lymphoma | |
| P3HR1 | + (II) | EBV-related Burkitt lymphoma | |
| LCL | + (III) | Primary B cells transformed with EBV | |
| Raji | + (III) | EBV-related Burkitt lymphoma | |
| T cell lines | Jurkat | - | Acute T lymphoblastic leukemia |
| MOLT4 | - | Acute T lymphoblastic leukemia | |
| SNT16 | + (II) | Chronic active EBV disease | |
| NK cell lines | KHYG1 | - | Aggressive NK cell leukemia |
| KAI3 | + (II) | Chronic active EBV disease |
- LCL, lymphoblastoid cell line.
Cell proliferation
Cells (2 × 105/mL) were cultured for 48 and 72 h in the presence of duvelisib or idelalisib at concentrations from 0.1 to 5 μmol/L. Viable cells were determined using a Countess™ Automated Cell counter (Invitrogen, Carlsbad, CA). Subsequent experiments were performed in triplicate at minimum.
Detection of PI3K/Akt signaling by immunoblotting
Collected cell extracts were diluted in sample buffer, and prepared for SDS-PAGE. Equal amounts of cellular protein extracts were loaded on 4–15% polyacrylamide gel. β-actin was used as a loading control. The separated protein bands were transferred to PVDF membranes, prior to antibody incubation. Membranes were incubated with primary antibodies at 4°C with gentle shaking, overnight. The primary antibodies were as follows: Akt, phospho-Akt (Thr308), PI3Kγ, PI3Kδ, and caspase-3 (Cell Signaling Technology, Beverly, MA), poly ADP ribose polymerase (PARP) and β-actin (Sigma-Aldrich, St. Louis, MO).
Apoptosis detection with Annexin V by flow cytometry
Apoptosis was assessed using an Annexin V-PE/7-AAD Apoptosis Detection Kit (BD Pharmingen Biosciences, San Diego, CA), following manufacturer's instructions. Briefly, cells (2 × 105/mL) were seeded in 24-well plates and treated with 5 μmol/L of duvelisib or DMSO for 48 h. Cells were then washed in PBS and incubated with Annexin V-phycoerythrin (PE) in a buffer containing 7-aminoactinomycin D (7-AAD). Apoptotic cells (7-AAD negative, PE Annexin V positive) were analyzed using flow cytometry.
Cell cycle analysis
Cells (2 × 105/mL) were treated with 5 μmol/L of duvelisib or DMSO for 48 h. Cells were then fixed with ice-cold 70% ethanol, and then washed with PBS. Fixed cells were re-suspended in 50 μg/mL propidium iodide (PI) solution (Sigma-Aldrich) with DNase-free RNase, and then analyzed using flow cytometry. Experiments were performed in triplicate.
Real-time RT-PCR
RNA was extracted from 1 × 106 cells from the culture medium with the QIAamp RNeasy Mini Kit (Qiagen, Hilden, Germany). Contaminating DNA was removed using the RNase-Free DNase set (Qiagen). The expression of two lytic (BZLF1 and glycoprotein 350/220) and six latent (EBNA1, EBNA2, LMP1, LMP2, EBER1, and BARTs) EBV genes was quantified by one-step multiplex real-time RT-PCR using QuantiFast Multiplex RT-PCR kits (Qiagen) and an Mx3000P instrument (Stratagene, La Jolla, CA) as described previously 36-38. The relative expression of EBV genes were determined by normalization to β2-microglobulin mRNA.
Results
Duvelisib suppresses growth of EBV-positive and -negative B cell lines
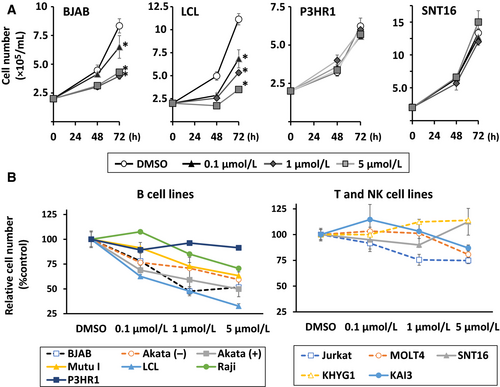
To determine the effects of duvelisib on EBV-negative B cell lines [BJAB and Akata (-)], EBV-positive B cell lines [Akata (+), Mutu I, LCL, Raji, and P3HR1], EBV-negative T cell lines (MOLT4 and Jurkat), EBV-positive T cell line (SNT16), EBV-negative NK cell line (KHYG1), and EBV-positive NK cell line (KAI3), cell lines were treated with 0.1–5 μmol/L of duvelisib and viable cells were counted after 48 and 72 h. We found that duvelisib inhibited cell growth in EBV-positive and -negative B cell lines, except in P3HR1, in a dose-dependent manner (Fig. 1A and B). Cell growth inhibition of EBV-negative T cell lines Jurkat, MOLT4, and EBV-positive NK cell line KAI3, was also observed at 1 or 5 μmol/L of duvelisib. However, no growth inhibition was observed in EBV-positive SNT16 and EBV-negative KHYG1. Overall, B cell lines were more susceptible to duvelisib than T and NK cell lines, and further experiments with duvelisib were focused on EBV-positive and -negative B cell lines.
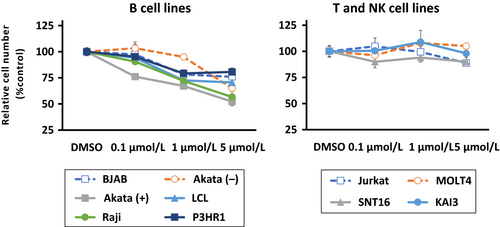
Effect of idelalisib on EBV-positive and negative cell lines
To confirm the effects of the selective PI3Kδ inhibitor idelalisib on B, T, and NK cell lines, 0.1–5 μmol/L of idelalisib was used to treat cells. Viable cells were counted after 72 h of treatment (Fig. 2). Growth of EBV-positive and –negative B cell lines was inhibited by idelalisib in a dose-dependent manner. On the other hand, no or modest growth inhibition was observed in T and NK cell lines.
Duvelisib inhibits activation of the PI3K/Akt signaling pathway in EBV-positive (+) and –negative (−) B cell lines
To confirm activation of the PI3K/Akt signaling pathway, we examined the status of PI3Kγ, PI3Kδ, and phospho-Akt (Thr308) in cell lines (Fig. 3). PI3Kγ expression was low in Raji cells, but was detected in all other cell lines tested. PI3Kδ was detected in all the cell lines that were tested. Duvelisib treatment decreased the expression level of PI3Kγ or PI3Kδ in Akata (−), Akata (+), and Jurkat. Conversely, the phosphorylated form of Akt was detected in all cell lines tested, indicating activation of Akt regardless of EBV status. Duvelisib treatment induced the inhibition of Akt phosphorylation in five of eight tested cell lines [BJAB, Akata (+), Mutu I, LCL, and Jurkat] (Fig. 3).

Duvelisib induces apoptosis in EBV-positive and -negative B cell lines
To investigate whether duvelisib induces apoptosis, Annexin V/7-AAD staining of various duvelisib-treated cells was evaluated. Duvelisib treatment increased the number of apoptotic cells (Annexin V-positive and 7-AAD-negative), compared to DMSO control treated cells in six of seven tested B cell lines (Fig. 4A). However, duvelisib did not induce apoptosis in B cell line P3HR1 and T cell lines (Jurkat and MOLT4).
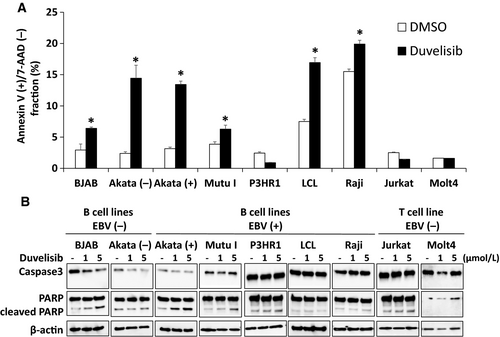
The cleavage of caspase-3 and PARP was also investigated in B and T cell lines to identify apoptosis after duvelisib treatment for 48 h. Duvelisib treatment induced cleavage of PARP in BJAB, Akata (+), and Mutu I (Fig. 4B). Decreased levels of full-length caspase-3 were observed in BJAB and Akata (−), whereas cleaved caspase-3 was not observed in any cell lines. The combined results of flow cytometry and immunoblotting suggest that duvelisib induces apoptosis in EBV-negative and -positive B cell lines, whereas it induces no apoptosis in T cell lines.
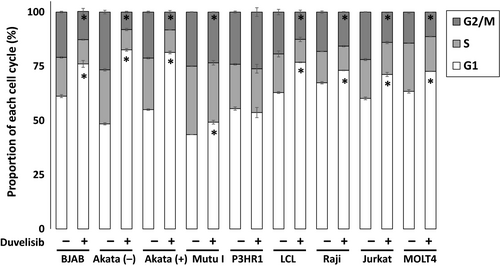
Duvelisib induces G1 cell cycle arrest in B and T cell lines
We next examined the effect of duvelisib on cell cycle distribution in EBV-positive and -negative B cells using flow cytometry analysis with PI staining. Duvelisib treatment induced an increase in cells in the G1 phase and a decrease in cells in the S and G2/M phases in all cell lines tested except P3HR1 (Fig. 5). These results indicate that duvelisib induces G1 cell cycle arrest in B and T cell lines resulting in inhibition of proliferation.
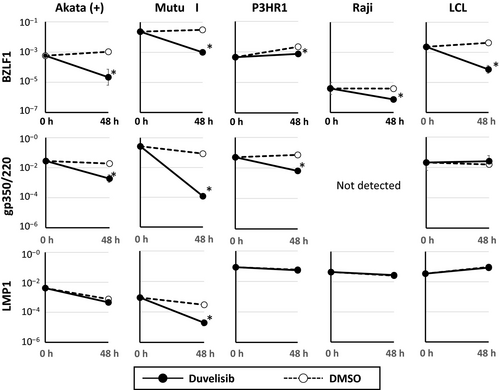
Duvelisib suppresses the expression of lytic EBV genes in B cell lines
We next investigated the effect of duvelisib on the expression of eight viral genes in B cell lines. The expression of BZLF1, which is an immediate-early lytic EBV gene, significantly decreased after duvelisib treatment in all EBV-positive B cell lines tested (Fig. 6). Furthermore, the expression of the late lytic EBV gene gp350/220 decreased significantly in Akata (+), Mutu I, and P3HR1. In contrast, the expression of LMP1, a latent oncogene of EBV, was decreased in Mutu I. However, this effect was not observed in other cell lines (Fig. 6). Duvelisib treatment did not induce remarkable change in the expression of the other five latent genes (EBNA1, EBNA2, LMP2, EBER1, and BARTs) (data not shown). Taken together, these results suggest that duvelisib suppresses the lytic cycle of EBV, but has little effect on the latent cycle.
Discussion
In this study, we evaluated the antitumor effects of duvelisib on EBV-associated lymphoma cell lines and found that B cell lines appeared to be more susceptible to duvelisib than T and NK cell lines regardless of the presence of EBV. It was expected that the presence of EBV might be associated with susceptibility to duvelisib because activation of the PI3K/Akt pathway via LMP1 is considered to be one of the key elements of EBV associated malignancies. Compared to EBV-negative Akata cell lines, inhibition of Akt phosphorylation by duvelisib was more clearly observed in EBV-positive cell lines. However, no remarkable differences were observed in susceptibility to duvelisib between EBV-positive and negative B cell lines. It might be possible that LMP1 or other EBV proteins have only a small effect on PI3K/Akt pathway activation in these cell lines.
LCL, which is classified as type III EBV latency, was the most susceptible to duvelisib among B cell lines tested. However, the association between latency pattern and susceptibility was not clear in this study. Interestingly, P3HR1 was insensitive to duvelisib despite that fact that it harbors an EBNA2-deficient EBV strain. One study has shown that EBNA2 upregulates Akt activation by inducing miR-21 expression 39. Although the phosphorylated form of Akt was detected in P3HR1 by immunoblotting, cellular proliferation of R3HR1 might be less dependent on the PI3K/Akt signaling pathway than other EBV-positive B cell lines.
Most of the B cell lines used in this study were cells originating from Burkitt lymphoma. To our knowledge, this is the first study to show the antitumor effects of duvelisib on Burkitt lymphoma cell lines because antitumor effects of duvelisib have been exclusively investigated in CLL 20, 21, 23. Regarding idelalisib, a selective PI3Kδ inhibitor, a recent study has shown that antiproliferative effects on EBV-positive and -negative Burkitt lymphoma cell lines (Namalwa and Ramos, respectively) were equivalent to its effects on CLL cell lines 40. While c-MYC deregulation is a hallmark of Burkitt lymphoma, synergy between constitutive PI3K/Akt signaling pathway activation and c-MYC has been shown. This suggests that the PI3K/Akt signaling pathway can be a therapeutic target in Burkitt lymphoma 41.
It was expected that duvelisib would have antitumor effects on T or NK cell lines as well as B cell lines because duvelisib is a dual inhibitor of PI3Kγ and PI3Kδ. Compared to idelalisib, a selective PI3Kδ inhibitor, duvelisib showed slightly more cell growth inhibition of T cell lines such as Jurkat or MOLT4. However, cell growth inhibition by duvelisib was modest in T or NK cell lines. Overall, the antitumor effects of idelalisib and duvelisib were similar in the cell lines that were tested. Furthermore, duvelisib did not induce apoptosis in T cell lines. On the other hand, G1 cell cycle arrest was observed in all B and T cell lines tested except P3HR1. Duvelisib treatment could inhibit T cell proliferation by inducing cell cycle arrest. However, its antitumor effects on T cells were limited because apoptosis was not induced.
We found that duvelisib treatment reduced the expression of BZLF1 and gp350/220 mRNA in EBV-positive B cell lines, suggesting that duvelisib suppresses the lytic cycle of EBV. In EBV-positive B cell lines, BCR signaling induces BZLF1 activation, and previous studies have shown that PI3K inhibitors such as wortmannin and idelalisib inhibit the EBV lytic cycle 42, 43. Our results are in line with these previous studies, and duvelisib may have specific effects on EBV-positive B cell lines. In general, the EBV latent cycle is associated with tumorigenesis, and among EBV latent proteins, LMP1 is considered to be a major EBV oncoprotein 5. Induction of the EBV lytic cycle by agents like proteasome inhibitors or histone deacetylase inhibitors could be therapeutically beneficial to EBV-associated malignancies 44, 45. Furthermore, previous reports have shown that induction of the EBV lytic cycle by chemotherapeutic agents enhanced antiviral nucleoside drug susceptibility to EBV positive tumor cells, suggesting that combination of chemotherapy and antiviral drug may provide a new therapeutic approach for EBV-associated malignancy 46, 47. Conversely, some reports have shown that the EBV lytic cycle may play a role in tumorigenesis in EBV-associated malignancies 48, 49. However, it is unclear whether inhibition of the EBV lytic cycle could be a therapeutic target of EBV-associated malignancies. In this study, suppression of the EBV lytic cycle was observed in P3HR1, which was insusceptible to duvelisib, and EBV-negative B cell lines showed similar susceptibility to duvelisib. It might be possible that inhibition of the EBV lytic cycle does not play an important role in antitumor effects of duvelisib on EBV-positive cell lines.
In conclusion, we demonstrated that the PI3K/Akt signaling pathway was activated in EBV-positive and -negative B cell lines. Further, duvelisib treatment induced apoptosis and cell cycle arrest. Although some progress has been achieved with treatments for EBV-associated lymphomas such as anti-CD20 antibody and adoptive EBV-specific cytotoxic T lymphocyte transfer, the effects of these new therapies are still restricted. Our results may indicate that duvelisib has therapeutic potential for the treatment of EBV-associated B cell lymphomas.
Acknowledgments
We thank Norio Shimizu (Tokyo Medical and Dental University, Tokyo, Japan) for the SNT16 cell line and Infinity Pharmaceuticals for providing duvelisib. This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan [to J.K. (17K10107)] and for the Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and Development [to H.K. (15ek0109098)].
Conflict of Interest
None declared.