Pooled analyses of randomized controlled trials on pyrotinib plus capecitabine and a rethink of the first-line options for HER2-positive relapsed or metastatic breast cancer
Graphical Abstract
This pooled analysis explored the possibility and potential of pyrotinib plus capecitabine as a first-line regimen in relapsed or metastatic HER2-positive breast cancer patients who received prior adjuvant or neoadjuvant chemotherapy with trastuzumab and discussed the potential treatment options for these patients, especially for the patients relapsed during or within 6 months after adjuvant trastuzumab.
Abbreviations
-
- BICR
-
- blinded independent central review
-
- HER2
-
- human epidermal growth factor receptor 2
-
- ORR
-
- overall response rate
-
- PFS
-
- progression-free survival
-
- RCT
-
- randomized controlled trial
-
- TKI
-
- tyrosine kinase inhibitor
Human epidermal growth factor receptor 2 (HER2)-positive breast cancer, which accounts for 15%–20% of breast cancers, is associated with aggressive tumor behavior and conferred a poor outcome before the era of HER2-targeted therapy. The advent of anti-HER2 antibodies (trastuzumab and pertuzumab), antibody–drug conjugates (ADCs, ado-trastuzumab emtansine and trastuzumab deruxtecan), and HER2-tyrosine kinase inhibitors (TKIs, neratinib and lapatinib), have dramatically improved the treatment response and survival of this aggressive subtype. Despite the significant clinical efficacy of trastuzumab in HER2-positive breast cancer patients, “initial” or “inherent” resistance to trastuzumab challenges its use in metastatic disease [1, 2]. The complementary mechanisms of action caused by the combination of different types of HER2-targeted agents provide a more comprehensive blockade of HER2 signaling, which may contribute to greater antitumor activity than monotherapy in HER2-positive tumor models [2]. Therefore, it is worthwhile exploring the optimal HER2-targeted treatment combination for HER2-positive metastatic breast cancer, especially for patients who relapsed during or within 6 months after adjuvant trastuzumab.
Pyrotinib is an oral, irreversible pan-ERBB TKI that potently inhibits epidermal growth factor receptor/HER1, HER2, and HER4. The efficacy and safety of pyrotinib plus capecitabine have been investigated in three randomized controlled trials (RCTs). The pivotal phase 2 trial [3] suggested that, compared with lapatinib plus capecitabine, pyrotinib combined with capecitabine showed an increase in the overall response rate (ORR) of more than 20% in HER2-positive metastatic breast cancer patients with prior taxanes, anthracyclines, and/or trastuzumab. In the PHOEBE [4] and the PHENIX [5] phase 3 trials, pyrotinib plus capecitabine showed a clinically and statistically significant improvement in progression-free survival (PFS) compared with lapatinib plus capecitabine and placebo plus capecitabine in HER2-positive metastatic breast cancer patients who received prior trastuzumab and taxanes. On the basis of these findings, pyrotinib has been approved by the National Medical Products Administration in China for treatment in combination with capecitabine in patients with relapsed or metastatic HER2-positive breast cancer previously treated with anthracyclines or taxanes. Because pertuzumab was not approved in China during the recruitment periods of the above three clinical trials of pyrotinib (between May 2015 and October 2018), none of these patients had access to pertuzumab before enrollment. The common design features of the three trials make it feasible to perform a pooled analysis to evaluate the possibility and potential of pyrotinib plus capecitabine as a first-line regimen in metastatic HER2-positive breast cancer patients who received prior adjuvant or neoadjuvant chemotherapy with trastuzumab and discuss the potential treatment options for these patients, especially those who relapsed during or within 6 months after adjuvant trastuzumab.
In the pooled analysis of the above three RCTs [3-5] (Supporting Information: Materials and Methods) to assess pyrotinib plus capecitabine in the first-line setting, a total of 145 female patients who received pyrotinib plus capecitabine as first-line therapy for HER2-positive metastatic breast cancer were analyzed: 51 patients from the PHOEBE phase 3 trial, 61 patients from the PHENIX phase 3 trial, and 33 patients from the pivotal phase 2 trial (Supporting Information: Table 1 and Figure 1). The median PFS was 12.4 months (95% confidence interval [CI], 11.1–not reached [NR]; Figure 2a) and the ORR was 72.4% (95% CI, 64.4%–79.5%) according to blinded independent central review (BICR) assessment. Regarding patients who underwent pyrotinib plus capecitabine as first-line therapy, 129 (89.0%) of them had prior trastuzumab as adjuvant therapy, and similar PFS benefits were observed in these patients when compared with the whole cohort of the pooled analysis (median, 12.5 months [95% CI, 11.1–20.7]; Figure 2b). Moreover, there were 20 (13.8%) trastuzumab-resistant (defined as having relapsed during or within 6 months after adjuvant trastuzumab) patients and 109 (75.2%) patients without the trastuzumab-resistant disease. Notably, there was no significant difference observed in PFS benefits per BICR between trastuzumab-resistant patients and those without the trastuzumab-resistant disease (median, 12.4 months [95% CI, 5.6–NR] vs. 13.8 months [95% CI, 11.1–20.7], one-sided stratified log-rank p = 0.201, Figure 2c).
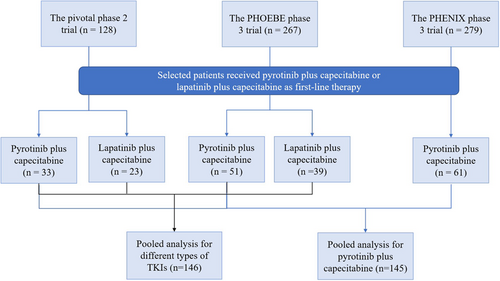
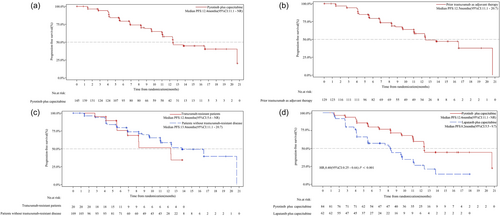
To compare different types of TKIs as first-line therapy, a total of 146 treatment-naive patients in the metastatic setting were assessed in the pooled analysis from the PHOEBE phase 3 [4] and the pivotal phase 2 [3] trials (Figure 1), including 84 (57.5%) patients who received pyrotinib plus capecitabine and 62 (42.5%) who received lapatinib plus capecitabine as first-line therapy. Among them, the median PFS was 12.4 months (95% CI, 11.1–NR) with pyrotinib plus capecitabine and 8.2 months (95% CI, 5.5–9.7) with lapatinib plus capecitabine, which presented a statistically significant improvement of 60% reduction in the estimated risk of disease progression or death in the pyrotinib group (hazard ratio, 0.40 [95% CI, 0.25–0.66]; one-sided stratified log-rank p = 0.0001) (Figure 2d).
In the pooled analysis of the three RCTs [3-5], the most common grade ≥3 adverse events (AEs) with pyrotinib plus capecitabine were diarrhea (29.7%), hand-foot syndrome (20.0%), decreased white blood cell count (7.6%), and hypertriglyceridemia (7.6%) (Supporting Information: Table 2), which was consistent with reports of previous clinical trials [3-5]. Because there was no prophylaxis for diarrhea in these three studies, the most common AE was diarrhea (96.6%). However, most diarrhea cases were grade 1/2 (66.9%), and no grade 4/5 diarrhea was reported in the pooled analysis (Supporting Information: Table 2). Overall, diarrhea was an unpleasant but manageable AE with pyrotinib combination therapy and could be generally alleviated with anti-diarrhea therapies, treatment interruption, or dose reduction. Among the three RCTs, only one patient (from the PHENIX phase 3 trial) reported permanent discontinuation of pyrotinib due to diarrhea.
Considering the observation from the pooled analysis for patients undertaking pyrotinib plus capecitabine as first-line therapy, 89.0% of the included patients who received prior trastuzumab in the adjuvant setting achieved similar PFS to the whole cohort. Among them, similar PFS benefits were observed in 20 trastuzumab-resistant patients compared with those in non-trastuzumab-resistant patients. Subgroup analyses suggested that prior use of trastuzumab may not influence the efficacy of TKIs for HER2-positive breast cancer, indicating different resistance mechanisms between monoclonal antibodies and small-molecule TKIs. Additionally, TKIs may induce the accumulation of HER2 at the surface of cancer cells, resulting in the enhancement of trastuzumab-mediated antibody-dependent cellular cytotoxicity [6]. A significantly increased pathological complete response rate was achieved by the combination of lapatinib plus trastuzumab for the neoadjuvant setting in the NeoALTTO study [7], and a significant survival benefit was gained by the combination of tucatinib plus trastuzumab for previously treated patients in the HER2CLIMB study [8]. Both of these results indicated that the combination of monoclonal antibodies and TKIs merits further investigation as HER2-targeted combined treatment options for HER2-positive breast cancer, even as first-line combination therapy. An ongoing phase 3 trial (NCT03863223) [9] is being conducted to compare the efficacy and safety of pyrotinib plus trastuzumab plus docetaxel versus placebo plus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer, which may provide another potential HER2-directed combination regimen.
The significantly prolonged PFS and overall survival achieved in the CLEOPATRA study [10] suggested the potent efficacy of the combination of two monoclonal antibodies, pertuzumab and trastuzumab, as first-line therapy for HER2-positive metastatic breast cancer. However, only 10.9% of the patients received prior adjuvant/neoadjuvant chemotherapy with trastuzumab [10]. Owing to the increased use of trastuzumab and pertuzumab in the neoadjuvant/adjuvant setting, the combination of pyrotinib or other pan-HER inhibitors with chemotherapy might be a proper selection as first-line therapy for HER2-positive metastatic breast cancer patients, especially those who relapsed during or within 6 months after neoadjuvant/adjuvant trastuzumab and pertuzumab potentially due to different resistance mechanisms between monoclonal antibodies and TKIs.
The current pooled analysis study and the ongoing trial (NCT03863223) [9] of the combination of pyrotinib plus trastuzumab may provide more choices for clinicians regarding first-line treatment selection for HER2-positive metastatic breast cancer patients who received prior trastuzumab in the neoadjuvant/adjuvant setting, especially trastuzumab-resistant patients. However, the sample size of this pooled analysis had limited power to enable solid conclusions to be drawn, and further studies exploring targeted therapy in HER2-positive metastatic breast cancer patients resistant to prior trastuzumab and pertuzumab in such settings could provide more information on first-line treatment options. Additionally, further translational work to investigate the proper biomarkers for HER2-directed combination regimens according to specific mechanisms in the blockade of HER2 signaling may help to identify appropriate patients who could most likely benefit from different HER2-directed treatments.
In summary, this pooled analysis indicated similar PFS benefits in trastuzumab-resistant HER2-positive metastatic breast cancer patients who received pyrotinib plus capecitabine as a first-line regimen. Further clinical research and translational work are warranted to investigate the appropriate treatment options for patients receiving prior adjuvant or neoadjuvant chemotherapy with trastuzumab, especially those who relapsed during or within 6 months after adjuvant trastuzumab.
AUTHOR CONTRIBUTIONS
Xiuwen Guan: data curation (lead); formal analysis (lead); investigation (lead); software (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review & editing (lead). Fei Ma: conceptualization (lead); data curation (lead); investigation (equal); methodology (lead); project administration (lead); resources (lead); supervision (lead); validation (lead); visualization (lead); writing – review & editing (lead). Binghe Xu: conceptualization (equal); data curation (equal); project administration (lead); resources (lead); supervision (lead); validation (lead); visualization (lead); writing – review & editing (supporting).
ACKNOWLEDGMENTS
We thank all the directors and study coordinators for presenting the referred clinical trials.
CONFLICT OF INTEREST
The authors declare no conflict of interest. Professor Fei Ma and Binghe Xu are members of the Cancer Innovation Editorial Board. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in the published article and supplementary files.