C-terminal variants of the P2X7 receptor are associated with prostate cancer progression and bone metastasis – evidence from clinical and pre-clinical data
Haiping Song and Hector Manuel Arredondo Carrera contributed equally
Abbreviations
-
- ATP
-
- adenosine triphosphate
-
- BPH
-
- benign prostatic hyperplasia
-
- BzATP
-
- Benzoylbenzoyl-ATP
-
- Cter
-
- C-terminal
-
- ec
-
- extracellular
-
- GDP
-
- guanosine diphosphate
-
- GTP
-
- guanosine triphosphate
-
- P2X7R
-
- P2X purinergic receptor 7
-
- PCa
-
- Prostate cancer
-
- WT
-
- wild-type
-
- ∆C
-
- C-terminal modified
Bone is the most common site for metastasis in prostate cancer (PCa), and identifying clinically useful biomarkers to predict the risk of currently incurable bone metastases is a major priority in PCa research.
Extracellular adenosine triphosphate (ATP) was shown to be a major biochemical constituent of the tumor microenvironment and regulates the tumor and host interactions by acting at P2 purinergic receptors. Among the P2 receptors, the ion channel P2X purinergic receptor 7 (P2X7R) has an unusually long C-terminus containing 200 amino acids, which forms the molecular basis for the unique bi-function of P2X7R promoting cancer cell proliferation or inducing membrane permeabilization and apoptosis, upon activation by a low or high concentration of ATP, respectively. A cysteine-rich region and a guanosine diphosphate or triphosphate (GDP/GTP)-binding site located in the C-terminus are pivotal for the structural rearrangement of the second transmembrane domain helix and the globular ballast underneath during the membrane pore permeabilization [1].
Among the 10 human P2X7R splice variants (P2X7RA-J), the P2X7RA variant is the full-length “wild-type (WT)” receptor, while the truncated P2X7RB variant lacks the intracellular C-terminal tail. This naturally occurring P2X7RB isoform maintains ATP-stimulated channel activity but not pore permeabilization [2, 3]. P2X7RB is upregulated in epithelial cancer cells [3] and associated with the trophic activity of primary bone cancer [2, 4, 5]. These findings have highlighted the importance of defining the role of P2X7R C-terminal truncated variants in cancer progression, particularly considering that data on P2X7R in PCa bone metastasis is lacking, whereas clinical evidence offers contradictory results [6, 7].
To clarify the role of P2X7R in PCa, we collected biopsies and surgical specimens from 77 patients with PCa and 9 patients with benign prostatic hyperplasia (BPH) from three local hospitals in Qingdao, Shandong, China. Among these PCa patients, 20 (26.0%) had bone metastases at the time of diagnosis, while 57 (74.0%) had no bone metastases (Supplementary Table S1). To distinguish the full-length WT P2X7RA and the C-terminus truncated P2X7RB splice variants, we used two antibodies specifically against different domains of the P2X7R, including anti-P2X7R-Cter (C-terminal) antibody against the P2X7RA-specific C-terminal tail, thus detecting the P2X7RA isoform, whilst the anti-P2X7R-ec (extracellular) antibody against the extracellular domain and detecting both P2X7RA and P2X7RB [4]. The detailed Methods can be found in the Supplementary file. Therefore, P2X7RA was defined as positive staining for both anti-P2X7R-Cter and anti-P2X7R-ec, while P2X7RB was positive for anti-P2X7R-ec but negative for anti-P2X7R-Cter (Figure 1A). Of the 66 P2X7R expressing PCa specimens (anti-P2X7R-ec-positive), 47 (71.2%) expressed P2X7RA, and 19 (28.8%) expressed P2X7RB. Comparing the Gleason scores, P2X7RB-positive cases were 8.2% higher than P2X7RA-positive cases (P = 0.047, Figure 1B). P2X7RB was not expressed in BPH specimens (0/9) but was expressed in 25.9% of PCa cases without bone metastases (14/54) and 41.7% of cases with bone metastases (5/12). The fraction of P2X7RB-positive cases were positively correlated with PCa progression (r = 0.997, P = 0.048, Figure 1C), based on the average Gleason scores of different disease stages (PCa without bone metastases = 8.0, bone metastatic PCa = 8.9, Supplementary Figure S1A). Immunoreactivity analysis showed no significant difference in P2X7RA expression level among specimens from the BPH group and groups of PCa with or without bone metastases (Supplementary Figure S1B-C), which was consistent with the retrospective analysis of clinical datasets (Supplementary Figure S1D-E). Therefore, C-terminus truncated P2X7RB variants, but not full-length P2X7RA, were positively associated with PCa progression and bone metastases.
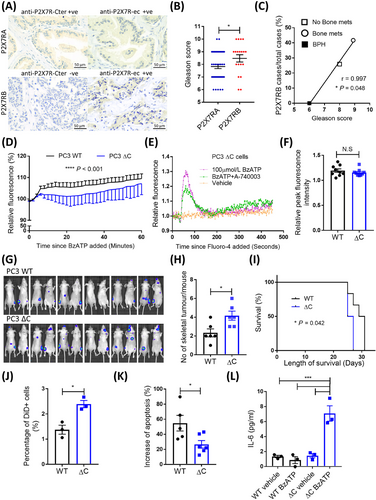
As P2RX7 exon 13 encodes 85% length of the entire C-terminus and is key to C-terminal functions, CRISPR/Cas9 technique was used to generate a frameshift after residue position 451 in exon 13 (∆C) (Supplementary Figure S2, S3), to understand how P2X7R C-terminal disruptions affect PCa cell biology. In WT PCa cells, exposure to 300 μmol/L P2X7R-specific agonist BzATP induced ethidium bromide uptake, suggesting that P2X7R mediated membrane permeabilization to large molecules, a unique property of the P2X7R C-terminus. While in ∆C cells, this was significantly reduced (P < 0.001, Figure 1D). To examine whether ∆C P2X7R is still functional in PCa cells, we tested the BzATP-induced calcium influx. BzATP was able to induce a calcium influx in PC3 ∆C cells, which was inhibited by the P2X7R antagonist A740003 (Figure 1E). There was no significant difference in BzATP-induced calcium influx peak between PC3 WT and ∆C cells (P = 0.388, Figure 1F). In addition, PC3 ∆C cells showed a significantly reduced presence of the P2X7R C-terminus at the protein level (Supplementary Figure S3F-I). These data have confirmed that our P2X7R ∆C resembled the unique characteristics of C-terminal truncated P2X7RB isoform, i.e., maintaining channel activity but not membrane pore permeabilization [2].
To test whether the disruption of P2RX7 exon 13 altered the metastatic ability of PCa cells, we tracked bone tumors formed by WT and ∆C PC3 cells following intra-cardiac injection (Figure 1G). Mice injected with ∆C cells had almost twice the skeletal tumor frequency (number of skeletal tumors/mouse) of the WT group (4.2 vs. 2.3, P = 0.016, Figure 1H), with a significant 4-day reduction in survival compared to the WT group (median survival: 26 vs. 30 days, P = 0.042, Figure 1I). However, there were no significant differences in the individual tumor size (Supplementary Figure S4A), number of non-skeletal tumors (Supplementary Figure S4B), or tumor-induced bone destruction determined ex vivo using micro-CT (Supplementary Figure S4C-F). These were consistent with data from cellular assays showing that disrupting P2X7R C-terminus did not impair proliferation (Supplementary Figure S5) and suggested that the pro-bone metastasis effects in C-terminal disrupted PCa cells were bone tropism related rather than tumor growth per se.
To provide an insight into what led to the enhanced metastatic potential of ∆C cells, we performed a metastasis-initiating cell assay [8] and found that PC3 ∆C cells had significantly increased numbers of bone tropic metastasis-initiating cells by 74.1% (P = 0.012, Figure 1J). We examined these cells’ response to stimulation of pore-inducing high concentration of extracellular BzATP (300 μmol/L) and showed a significant reduction of induced apoptosis in PC3 ∆C cells (P = 0.030, Figure 1K) but an increased secretion of pro-metastatic cytokine IL-6 (Figure 1L). The enhanced presence of the metastasis-initiating cell subpopulation in P2X7R ∆C cells was probably linked to their ability to escape apoptosis. The P2X7R C-terminus, with the presence of palmitoylated cysteine residues, could be inserted into the plasma membrane and facilitated pore formation by preventing cholesterol interaction with P2X7R [9]. Disruption of the C-terminus, like in the P2X7RB isoform or ∆C cells, could significantly reduce the induction of apoptosis in cells under the stimulation of pore-inducing high concentration of extracellular nucleotides (e.g., 100-500 μmol/L extracellular ATP within solid tumors) [10]. Furthermore, when stimulated by extracellular BzATP, only ∆C cells secreted IL-6, a pro-inflammatory cytokine with strong pro-metastatic effects on bone metabolism, tumor cell survival, and angiogenesis. These findings warrant further investigation to clarify the exact regulatory mechanisms determining the metastatic fate of P2X7R ∆C cells.
In conclusion, using both clinical and pre-clinical data, we showed that the disruption of the C-terminus of P2X7R was positively associated with PCa progression and bone metastasis. The C-terminal truncated P2X7RB isoform, rather than the full-length P2X7RA, could potentially be used as a prognostic marker to predict PCa bone metastasis.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Haiping Song: Investigation, Formal analysis, Visualization and Funding acquisition; Hector Manuel Arredondo Carrera: Investigation, Formal analysis and Visualization; Alexandria Sprules: Investigation, Validation and Methodology; Ying Ji: Investigation and Validation; Tongsong Zhang: Investigation and Validation; Jiepei He: Investigation; Eleanor Lawrence: Investigation; Alison Gartland: Writing - Review & Editing; Jian Luo: Writing - Review & Editing and Funding acquisition; Ning Wang: Conceptualization, Writing - Original Draft, Supervision, Project administration, Funding acquisition.
ACKNOWLEDGMENTS
These studies received supports from the Royal Society/the National Natural Science Foundation of China (NSFC) and Qingdao Science and Technology Bureau Research Fund. We also wish to thank the following colleagues for their excellent technical support: Dr. Bin Ding, Pathology Department, Qingdao Tumor Hospital, China; Dr. Changqing Jiang, Pathology Department, Qingdao Municipal Hospital, China; Dr. Yunqing Chen, Pathology Department, the Affiliated Hospital of Medical College, Qingdao University, China; and Dr. Karan M Shah, Department of Oncology & Metabolism, The University of Sheffield, UK.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING INFORMATION
This research was funded by the International Exchange Scheme – Cost Share Programme, jointly sponsored by the Royal Society, UK (grant number IEC∖NSFC∖170113) and the National Natural Science Foundation of China (NSFC) (grant number 8171101485). This research was also funded by the Qingdao Science and Technology Bureau Research Fund, China, grant number 18-6-1-98-nsh.
AVAILABILITY OF DATA AND MATERIALS
Raw data for retrospective analysis can be accessed through the Gene Expression Omnibus (GEO) database (Accession Number: GDS1439 and GDS3289). The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
ETHICS APPROVAL
For clinical samples, written informed consent was obtained from all patients, and the study was approved by the Ethical Review Committee of the Qingdao Tumor Hospital to ensure that rights and privacy of patients were protected (Approval Number: [Y]KY20200001). For animal studies, all procedures complied with the UK Animals (Scientific Procedures) Act 1986 and were reviewed and approved by the local Research Ethics Committees of the University of Sheffield under Home Office project license 70/8799 (Sheffield, UK).




