Breast cancer: an up-to-date review and future perspectives
Abstract
Breast cancer is the most common cancer worldwide. The occurrence of breast cancer is associated with many risk factors, including genetic and hereditary predisposition. Breast cancers are highly heterogeneous. Treatment strategies for breast cancer vary by molecular features, including activation of human epidermal growth factor receptor 2 (HER2), hormonal receptors (estrogen receptor [ER] and progesterone receptor [PR]), gene mutations (e.g., mutations of breast cancer 1/2 [BRCA1/2] and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha [PIK3CA]) and markers of the immune microenvironment (e.g., tumor-infiltrating lymphocyte [TIL] and programmed death-ligand 1 [PD-L1]). Early-stage breast cancer is considered curable, for which local-regional therapies (surgery and radiotherapy) are the cornerstone, with systemic therapy given before or after surgery when necessary. Preoperative or neoadjuvant therapy, including targeted drugs or immune checkpoint inhibitors, has become the standard of care for most early-stage HER2-positive and triple-negative breast cancer, followed by risk-adapted post-surgical strategies. For ER-positive early breast cancer, endocrine therapy for 5-10 years is essential. Advanced breast cancer with distant metastases is currently considered incurable. Systemic therapies in this setting include endocrine therapy with targeted agents, such as CDK4/6 inhibitors and phosphoinositide 3-kinase (PI3K) inhibitors for hormone receptor-positive disease, anti-HER2 targeted therapy for HER2-positive disease, poly(ADP-ribose) polymerase inhibitors for BRCA1/2 mutation carriers and immunotherapy currently for part of triple-negative disease. Innovation technologies of precision medicine may guide individualized treatment escalation or de-escalation in the future. In this review, we summarized the latest scientific information and discussed the future perspectives on breast cancer.
Abbreviations
-
- ALN
-
- axillary lymph node
-
- ALND
-
- axillary lymph node dissection
-
- AI
-
- artificial intelligence
-
- APBI
-
- accelerated partial-breast irradiation
-
- BARD1
-
- BRCA1 associated RING domain 1
-
- BCI
-
- Breast Cancer Index
-
- BCS
-
- breast-conserving surgery
-
- BRCA1/2
-
- breast cancer 1/2
-
- BRIP1
-
- BRCA1 interacting helicase 1
-
- CBR
-
- clinical benefit rate
-
- CDH1
-
- cadherin 1
-
- CHEK2
-
- checkpoint kinase 2
-
- CI
-
- confidence interval
-
- CPS
-
- combined positive score
-
- CTS
-
- including clinical treatment score
-
- ctDNA
-
- circulating tumor DNA
-
- ddPCR
-
- droplet digital PCR
-
- DFS
-
- disease-free survival
-
- EBCTCG
-
- Early Breast Cancer Trialists Collaborative Group
-
- EFS
-
- event-free survival
-
- ER
-
- estrogen receptor
-
- ESR1
-
- estrogen receptor 1
-
- ET
-
- endocrine therapy
-
- FANCC
-
- fanconi anemia complementation group C
-
- FDA
-
- Food and Drug Administration
-
- HDAC
-
- histone deacetylase
-
- HER2
-
- human epidermal growth factor receptor 2
-
- HR
-
- hormone receptor
-
- IDFS
-
- invasive disease-free survival
-
- IHC
-
- immunohistochemistry
-
- MBC
-
- metastatic breast cancer
-
- MRI
-
- magnetic resonance imaging
-
- NET
-
- neoadjuvant endocrine therapy
-
- NF1
-
- neurofibromatosis type 1
-
- NOS
-
- not otherwise specified
-
- OFS
-
- ovarian function suppression
-
- ORR
-
- overall response rate
-
- OS
-
- overall survival
-
- PALB2
-
- partner and localizer of BRCA2
-
- PAM 50
-
- Prosigna
-
- PARP
-
- Poly (ADP-ribose) polymerase
-
- pCR
-
- pathological complete response
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L1
-
- programmed death-ligand 1
-
- PEPI
-
- Preoperative Endocrine Prognostic Index
-
- PFS
-
- progression-free survival
-
- PIK3CA
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
-
- PI3K
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase
-
- PR
-
- progesterone receptor
-
- RAD51C
-
- RAD51 homolog C
-
- RAD51C
-
- RAD51 Paralog C
-
- RAD51D
-
- RAD51 Paralog D
-
- RT
-
- radiotherapy
-
- RR
-
- relative risk
-
- STK11
-
- serine/threonine kinase 11
-
- SERM
-
- selective estrogen receptor modulator
-
- SLN
-
- sentinel lymph node
-
- SLNB
-
- sentinel lymph node biopsy
-
- TAD
-
- targeted axillary dissection
-
- T-DXd
-
- trastuzumab deruxtecan
-
- TIL
-
- tumour-infiltrating lymphocyte
-
- TNBC
-
- triple-negative breast cancer
-
- Trop-2
-
- trophoblast cell-surface antigen 2
-
- WHO
-
- World Health Organization
1 INCIDENCE AND EPIDEMIOLOGY OF BREAST CANCER
Female breast cancer has overtaken lung cancer as the most common diagnosed cancer worldwide. The estimated new breast cancer cases reached 2.3 million in 2020, accounting for 11.7% of all new cancers, and 684,996 cases died of it [1]. In China, breast cancer was the most common malignancy among women, with an estimated number of 306,000 new cases occurring in 2016 [2]. The incidence of breast cancer has increased since the widespread uptake of mammography screening and continues to increase with the aging of the population.
Globally, death rates for female breast cancer were conspicuously higher in transitioning countries versus transitioned countries (15.0 per 100,000 vs. 12.8 per 100,000) [1]. In most western countries, the breast cancer mortality rate has decreased in recent years, owing to modern treatment strategies and earlier detection [3, 4]. In China, breast cancer ranked first among causes of cancer deaths in women aged 15-44 years, and there was still a rising trend of mortality rates for breast cancer [2].
There is a disparity in incidence by age between China and western countries. In the western population, only approximately a quarter of breast cancers are diagnosed before 50 years old, and only <5% before the age of 35. While in China, about two in three are diagnosed between 40-59 years old. The difference is also seen with mean age values. The mean age at diagnosis for Chinese breast cancers is 49, which is considerably different from the western countries, where the mean ages of diagnosis are 60 (Australia) and 61 (the U.S.), respectively [5].
The major risk factors of breast cancer include: reproductive and hormonal risk factors (early age at menarche, later age at menopause, advanced age at first birth, fewer number of children, less breastfeeding, menopausal hormone therapy, and oral contraceptives), lifestyle risk factors (excess body weight, physical inactivity, and alcohol intake), as well as genetic predisposition (germline mutations of high-penetrance genes such as breast cancer 1/2 [BRCA1/2], partner and localizer of BRCA2 [PALB2], ATM, checkpoint kinase 2 [CHEK2], RAD51 homolog C [RAD51C], BRCA1 associated RING domain 1 [BARD1], TP53, etc.) [6-8]. Male breast cancers account for 1% of all breast cancer cases. Family history and genetic predisposition, hormonal imbalances caused by clinical disorders (such as gynaecomastia and cirrhosis), and radiation exposure may contribute to the occurrence of breast cancer in males [9].
2 BREAST CANCER SCREENING AND PREVENTION
Recommendations for breast cancer screening should be based on risk stratification. Women are classified into two basic groups: average risk and increased risk (e.g., BRCA1/2 mutations). Mammography remains the most important imaging modality for screening, as it is the only one that has demonstrated a mortality reduction [10]. Digital mammography generates an electronic image that allows to store and process by a computer, thereby increasing the cancer detection rate, particularly in younger women with dense breasts [11-13]. Adding ultrasound screening to mammography in women with dense breasts increased cancer detection and elevated recall and biopsies of the benign breast. A large prospective screening study showed that adding ultrasound to mammography identified an additional 4.3 cancers per 1000 cases in women with dense breasts and a high risk of breast cancer [14]. In women with dense breasts, the sensitivity of mammography was found to be 50.0%, and that of mammography plus ultrasound was 77.5% [14].
Contrast-enhanced breast magnetic resonance imaging (MRI) is highly sensitive to breast cancer detection, showing a sensitivity of 90%-93%, which is higher than the sensitivity of 48%-63% for mammography and ultrasound combined in women at high risk [15, 16]. However, the specificity of MRI screening is lower, resulting in a high false-positive rate [17]. The current evidence does not support the use of breast MRI as a screening modality in women at average risk, whereas the benefits of screening MRI for early detection in women with a high risk of breast cancer, e.g., those with a known genetic predisposition, or strong family history, has been demonstrated in several trials [18-22]. Some countries recommend MRI screening in women at elevated risk of breast cancer [23].
Women with increased breast cancer risk have various options to lower their risk, including surgery, medication and lifestyle modification. Bilateral mastectomy is the most effective way of reducing breast cancer risk and is recommended for women at substantially high risk (e.g., those with high-risk breast cancer gene mutation or prior history of thoracic radiation before 30 years old) [24]. BRCA1 and BRCA2 mutation carriers should be counseled to consider bilateral salpingo-oophorectomy to reduce the risk of malignancies of the ovary and fallopian tube by the age at which the risk of these cancers increases above that of the general population, which is the late 30s (for BRCA1 mutation carriers) and late 40s (for BRCA2 mutation carriers) [25]. Medication prevention recommended by international guidelines includes selective estrogen receptor modulators (SERMs), tamoxifen (IBIS-I and NSABP-P1 trials), raloxifene (STAR trial), or an aromatase inhibitor (IBIS-II trial) [26-29]. None of these agents have been reported to reduce breast cancer mortality and all of which are only capable of preventing estrogen receptor (ER)-positive breast cancer. A recent randomized trial suggested that tamoxifen 5 mg daily for 3 years might be as effective in preventing the recurrence of breast intraepithelial as the usual dose of 20 mg daily for 5 years, with limited toxicities [30]. Based on this result, tamoxifen 5 mg daily for 3 years could be considered an alternative for women who could not tolerate the usual dose of tamoxifen [31].
3 HEREDITARY BREAST CANCERS AND SYNDROMES
Deleterious, hereditary mutations explain 8%-10% of all breast cancers. Half of these cases are associated with BRCA1/2 mutations, while the remaining are associated with less prevalent and fewer penetrant variations [32-35]. Germline mutations in BRCA1/2 account for about 15%-20% of all triple-negative breast cancers (TNBCs) [36]. In human epidermal growth factor receptor 2 (HER2)-negative, hormonal receptor-positive breast cancer cases with high-risk factors such as family history, BRCA1 or BRCA2 mutation carriers can account for 10%-15% [36]. Additionally, 0.6%-3.9% of familial breast cancers are associated with PALB2 germline mutations [36, 37]. BRCA1-associated breast cancers are often present with a triple-negative phenotype (70%-85%), while ER-positive, HER2-negative cases are more common with BRCA2 and PALB2 mutation carriers [38].
Population-based studies have classified several deleterious gene mutations into high, moderate or low penetrance groups. High penetrance genes confer a lifetime breast cancer risk of more than 3-fold relative to the general population, including BRCA1/2, PALB2, and TP53. Moderate penetrance genes (e.g., BARD1, CHEK2, cadherin 1 [CDH1], serine/threonine kinase 11 [STK11]) result in 2- to 3- fold increased breast cancer risk, and low penetrance genes (1-to 2- fold risk) include ATM, BRCA1 interacting helicase 1 (BRIP1), neurofibromatosis type 1 (NF1), RAD51 Paralog C (RAD51C), RAD51 Paralog D (RAD51D), and Fanconi anemia complementation group C (FANCC) [32, 34]. The degree of penetrance in mutated genes and age at genetic diagnosis influence the prophylactic surgery recommendations [39]. Generally, consideration of risk-reducing surgery is more favored for women harboring high penetrance gene mutations, and surveillance without prophylactic surgery could be a reasonable option for women with fewer penetrant mutations [39].
Testing for germline BRCA1/2 mutations in HER2-negative metastatic breast identifies candidates who might benefit from treatment of poly (ADP-ribose) polymerase (PARP) inhibitors or platinum drugs [40, 41]. The addition of platinum to standard chemotherapy confers improved pathological complete response (pCR) rate in neoadjuvant trials, although most of these trials were not sufficiently powered to indicate long-term survival outcomes [42, 43]. Data from the OlympiA trial showed a significant reduction in recurrence rate with adjuvant olaparib in BRCA1/2 mutation associated and HER2-negative breast cancer [44]. Based on these data, genetic testing for patients who meet the OlympiA criteria to identify candidates for adjuvant olaparib treatment is recommended.
4 SUBSETS AND MOLECULAR PATHOLOGY
Pathological diagnosis of breast cancer should be based on tissue obtained from a core needle biopsy and established according to the World Health Organization (WHO) classification [45]. The most common histological tumor types are invasive carcinoma of the breast, not otherwise specified (NOS, also called ductal carcinoma; 70%-75%) and lobular carcinoma (12%-15%) [45]. There are also other 18 rare subtypes, accounting for 0.5% to 5% of all breast tumors. The pathological assessment should include the histological type, grade, immunohistochemistry (IHC) evaluation of ER, progesterone receptor (PR) status and HER2 expression or HER2 gene amplification [46]. The proliferation marker Ki67 index may supply additional useful information. Standardized diagnostic evaluation of these markers is essential [47, 48].
Breast cancer is a highly heterogeneous disease. For prognostic prediction and treatment decision-making, breast tumors are classified into surrogate intrinsic subtypes, as defined by IHC testing of ER, PR, HER2, and Ki67 status [49, 50]. Luminal A-like tumors are those with low grade, strongly ER-positive/PgR-positive, HER2-negative, and low proliferative index. Luminal B-like tumors are ER-positive but may have low degrees of PR expression or have a higher proliferative index or positive HER2 expression. Other subtypes include HER2-positive and TNBC [49, 50]. In addition to pathological assessment, gene expression profiles, such as MammaPrint, Oncotype DX Recurrence Score, Prosigna (PAM 50), EndoPredict and Breast Cancer Index (BCI), may provide additional information to classify breast tumors based on different prognosis and benefit to adjuvant systemic treatment (Table 1) [48, 51].
| Age/menopausal status | Lymph node status | Biomarkers | Recommendations [51] |
|---|---|---|---|
| Premenopausal or age ≤ 50 years | Node-negative | Oncotype DX (21-gene recurrence score, 21-gene RS) |
For patients with RS ≥26, chemotherapy and endocrine therapy should be offered. For those with RS 16∼25, chemotherapy and endocrine therapy may be offered. |
| 1-3 positive nodes | Insufficient evidence to recommend | N/A | |
| ≥4 positive nodes | Insufficient evidence to recommend | N/A | |
| Postmenopausal or age > 50 years | Node-negative | Oncotype DX | For patients with RS ≥26, chemotherapy and endocrine therapy should be offered. |
| MammaPrint (70-gene signature) |
For patients with high clinical risk, MammaPrint test may be used to guide decisions for adjuvant endocrine and chemotherapy. For patients with low clinical risk, evidence is insufficient to recommend its routine use. |
||
| EndoPredict (12-gene risk score) | The clinician may use the EndoPredict test to guide decisions for adjuvant endocrine and chemotherapy. | ||
| Prosigna (PAM50) | The clinician may use the Prosigna to guide decisions for adjuvant systemic chemotherapy. | ||
| BCI | For patients treated with 5 years of primary endocrine therapy without evidence of recurrence, BCI may be offered to guide extended endocrine therapy decisions. | ||
| 1-3 positive nodes | Oncotype DX | For patients with RS ≥26, chemotherapy and endocrine therapy should be offered. | |
| MammaPrint |
For patients with high clinical risk, MammaPrint may be used to guide decisions for adjuvant endocrine and chemotherapy. For patients with low clinical risk, evidence is insufficient to recommend its routine use. |
||
| EndoPredict | The clinician may use the EndoPredict test to guide decisions for adjuvant endocrine and chemotherapy. | ||
| BCI | For patients treated with 5 years of primary endocrine therapy without evidence of recurrence, BCI may be offered to guide extended endocrine therapy decisions. | ||
| ≥4 positive nodes | Insufficient evidence to recommend | N/A |
- Abbreviations: RS, recurrence score; BCI, breast cancer index; N/A, not applicable.
Tumor-infiltrating lymphocyte (TIL) in the tumor and stroma has been demonstrated to have prognostic and predictive value for response to treatment in TNBC and HER2-positive breast cancer [52, 53]. In many neoadjuvant clinical trials, TIL has been identified as a predictor of pCR after chemotherapy [53]. IHC testing for programmed death-ligand 1 (PD-L1) is recommended in metastatic TNBC because its level can discriminate patients who can benefit from checkpoint inhibitors, but the predictive value of PD-L1 was not indicated in early breast cancer [54-56]. In ER-positive, HER2-negative metastatic breast cancer, somatic mutations of phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) are predictive of response to phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitors [57]. In HER2-positive breast cancer, the mutation status of PIK3CA is associated with response to anti-HER2 targeted therapy [58, 59]. Proposed biomarkers that need to be tested in metastatic breast cancer (MBC) patients are shown in Figure 1 [60].
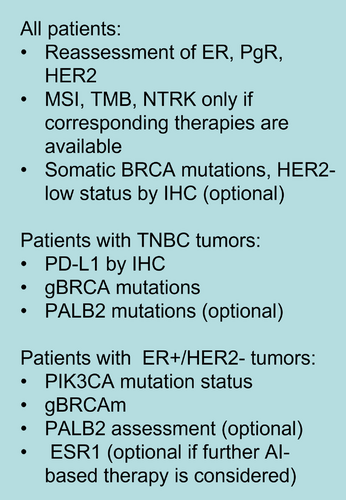
5 NEOADJUVANT THERAPY RATIONALE
It has been shown in randomized clinical trials that systemic chemotherapy given before or after surgery results in no differences in long-term outcomes among breast cancer patients [61, 62]. The conventional clinical advantages of preoperative or neoadjuvant systemic therapy include tumor down-staging, which can improve surgical outcomes. Preoperative therapy may turn inoperable tumors into operable ones and allows surgical de-escalation in the breast and axilla in patients with early-stage breast cancer. Approximately 40% of patients with HER2-positive and triple-negative tumors initially requiring mastectomy can be converted to breast-conserving surgery (BCS) candidates [63, 64]. Further, neoadjuvant systemic treatment may offer prognostic information based on the extent of treatment response, which can also identify patients with residual disease who may require additional adjuvant therapy. Achieving a pCR after neoadjuvant chemotherapy is predictive of significantly better disease-free survival (DFS) and overall survival (OS) in early breast cancer, and the correlation was most pronounced in triple-negative cancer and HER2-positive disease [64-66]. This observation has led to studies exploring the use of additional adjuvant therapy agents in patients with residual disease after standard neoadjuvant therapy. In the CREATE-X trial, the addition of capecitabine in the adjuvant setting improved DFS and OS in patients with HER2-negative breast cancer who did not achieve pCR after standard anthracycline and taxanes-containing preoperative therapy. The benefit of this approach was mainly seen in patients with triple-negative diseases [67]. In the KATHERINE trial, patients without pCR who switched from antibody-based HER2 targeted neoadjuvant therapy to the antibody-drug conjugate trastuzumab emtansine had significantly improved DFS than those who continued anti-HER2 antibody treatment (from 77%, with trastuzumab to 88%, with trastuzumab emtansine). The benefits are homogenous across subgroups, regardless of the extent of residual disease and ER status [68]. Based on the evidence, neoadjuvant therapy is considered as the preferred treatment strategy for patients with triple-negative or HER2-positive tumors at stage II or III.
6 EARLY BREAST CANCER: LOCAL-REGIONAL TREATMENT
6.1 Surgery
BCS is the optimal surgical option for early breast cancer patients. Oncoplastic techniques to achieve good cosmetic outcomes may be used when needed. BCS is contradicted for patients unable to achieve negative margins and have contraindications to radiotherapy (RT). BCS could also be an option for patients with a multifocal and multicentric disease if R0 resection is confirmed [69]. For breast surgery, “no ink on tumor” remains the accepted standard for negative margins. The 10-year locoregional recurrence rates with BCS followed by RT are approximately 2% to 3% for ER-positive and HER2-positive tumors and 5% for TNBC, which are similar to those seen after mastectomy [70, 71]. Post-surgical systemic therapy also contributes to the locoregional recurrence risk reduction [72]. For women who require a mastectomy, breast reconstruction could be an option. The optimal reconstruction technique should be discussed individually, considering anatomic, treatment and patient-related factors and preferences.
Axillary lymph node (ALN) dissection is associated with lymphoedema in up to 25% of women after surgery; the occurrence rate of lymphoedema dropped to below 10% in women receiving sentinel lymph node biopsy (SLNB) [73]. The randomized trial ACOSOGZ0011 has shown non-inferior locoregional recurrence or survival when treated with SLNB alone for patients with T1-2 cN0 status and metastasis in 1-2 sentinel lymph nodes (SLNs; who received BCS, tangential adjuvant RT including part of the axilla and adjuvant systemic therapy) [74, 75]. This observation was consistent with other three randomized trials [76-78]. Therefore, for patients with limited SLN involvement who are planning to receive tangential breast RT and systemic adjuvant therapy and meet the criteria of these randomized trials, ALN dissection (ALND) can be avoided. For patients who do not meet the trials’ criteria, ALND might be considered. Another option for patients with cN0 and SLN metastases is axillary RT without ALND, as demonstrated by the AMAROS trial [78].
Neoadjuvant chemotherapy could convert patients presenting with clinical N1 axilla to clinically negative axilla (cN0). Patients with clinically positive axilla after neoadjuvant chemotherapy should be treated with ALND, while those with cN0 status are potential candidates for SLNB. In patients with cN0 before neoadjuvant chemotherapy, the false-negative rate (6%-7%) and nodal recurrence rate (<1.5%) for SLNB after neoadjuvant therapy are similar to those seen in patients who receive primary surgery [79-82]. Four prospective trials evaluated the accuracy of SLNB in patients with initial positive cN status who received neoadjuvant treatment [82-85]. Results showed that false-negative rates were relatively high in patients who retrieved one or two SLNs, but the rate dropped to <10% in those who had three SLNs retrieved [82-85]. In a meta-analysis including 1921 patients with biopsy-confirmed axillary metastases, the SLN identification rate was 90%, and the false-negative rate was 14% after primary systemic therapy, which fell to 4% in those removed more than 3 SLNs [86]. Targeted axillary dissection (TAD) offers an alternative to increasing the accuracy of SLNB after neoadjuvant therapy [87, 88]. TAD involves SLNB and dissection of core-needle biopsy-proven and marked positive target lymph node. The effect of TAD on DFS is still awaiting. Generally, for patients without residual nodal disease, when the initially targeted and marked nodes or at least three SLNs are identified and removed, ALND after primary systemic therapy could be spared [89, 90].
6.2 RT
Postoperative RT is recommended for patients receiving BCS or mastectomy (with risk factors). A meta-analysis by the Early Breast Cancer Trialists Collaborative Group (EBCTCG) showed a significant reduction in the 10-year risk of recurrence in patients who received whole breast irradiation after BCS compared with those who did not (19% vs. 35%; relative risk [RR] = 0.52, 95% confidence interval [CI] = 0.48-0.56) [91]. A reduction of 15-year risk of breast cancer-related mortality of 4% was also observed (21% vs. 25%; RR = 0.82, 95% CI = 0.75-0.90). RT after mastectomy and ALND reduced the 10-year risk of recurrence by 10% and the 20-year risk of breast cancer mortality by 8% [92]. The benefit of post-mastectomy radiation is seen in patients with 1 to 3 involved ALNs even when adjuvant systemic treatment was administrated. Therefore, post-mastectomy chest wall irradiation should be considered for routine use in patients with 1-3 involved ALNs.
The importance of regional nodal irradiation after lumpectomy and mastectomy in node-positive patients is supported by results from randomized trials and meta-analyses [74, 93–96]. In addition, in selected patients with metastases in the SLN, axillary RT could be an alternative treatment when de-escalating axillary surgery is planned [76, 78].
Traditionally, doses used for adjuvant local/regional irradiation have been 45-50 Gy in 25-28 fractions over 5 weeks. Two pivotal trials (Ontario trial and START B trial) compared moderate hypofractionation (40-42.5 Gy in 15-16 fractions over 3 weeks) with 50 Gy in 25 fractions for 5 weeks, which demonstrated comparable 5-year local recurrence rates and normal tissue effects between the two schedules [97, 98]. Long-term follow-up confirmed the effectiveness and safety of moderate hypofractionated for whole breast irradiation, which consequently became the standard of care for most patients [99-101]. Subsequent FAST-Forward randomized trial indicated that ultra-hypofractionated (26 Gy in five fractions) irradiation to the breast or chest wall is non-inferior in local control rates compared with the 3-week schedule and showed similar normal tissue effect at 5 years [102]. The FAST-Forward main cohort did not include nodal irradiation, and the FAST-Forward nodal substudy result is not yet reported. Therefore, five fraction schedules for nodal RT should not yet be taken as a standard of care. Long-term follow-up of this trial and further reports from other prospective studies that are expected to add evidence to the ultrafast 1-week hypofractionation schedule will be important.
Accelerated partial-breast irradiation (APBI) is an appealing approach to shorten the treatment time and reduce treatment volumes. The rationale of APBI is based on the perception that most local failures occur close to the primary breast tumor site. Partial breast irradiation can be administrated through various techniques, including external beam RT, intra-operative RT and brachytherapy [103-106]. Studies comparing the effectiveness of different APBI approaches with whole breast irradiation showed that low-risk patients appear to be the optimal population for APBI treatment, considering the balance between benefit and risk [107-111]. More and long-term results of several past and ongoing prospective randomized APBI trials are awaited.
7 EARLY BREAST CANCER: SYSTEMIC TREATMENT
7.1 Endocrine therapy (ET)
Patients with ER- or PR-positive should receive adjuvant ET for 5-10 years. Options include tamoxifen (for pre- and post-menopausal women) or a steroidal (exemestane) or non-steroidal (letrozole or anastrozole) aromatase inhibitor (in postmenopausal women). Initial adjuvant endocrine treatment with an aromatase inhibitor significantly reduced the absolute risk of reduction and improved DFS compared with tamoxifen at 10 years in postmenopausal women [112, 113]. Several trials studied the sequential use of tamoxifen for 2 to 3 years, followed by an aromatase inhibitor [112]. A meta-analysis of the EBCTCG showed that 5-year adjuvant ET, including artificial intelligence (AI), was superior to tamoxifen monotherapy in reducing recurrence rate and breast cancer death, either by continuous or sequential use [114]. ER-positive breast cancers are characterized by late recurrence. Recurrences can occur from 5 to 20 years after surgery, correlating with the primary tumor, nodal status, and histological grade [115]. This has led to multiple trials investigating the benefit of extended ET for up to 15 years (Table 2) [116-121]. Results show that continuation of ET after 5 years decreases the risk of recurrence in patients at high risk (e.g., node-positive or high genomic score) [116]. Some prognostic tools may help assess the risk of late recurrence, including clinical treatment score (CTS5) and multigene signatures [122, 123]. By now, only the BCI assay has been reported to be predictive of benefit for extended ET independent of the risk of late recurrence [124, 125]. Extended therapy should be considered for patients with higher risks and balance potential benefits, toxicity, and impaired quality of life.
| Clinical trial | Number of cases | Study design | Main finding |
|---|---|---|---|
| ATLAS [120] | 12894 | TAM 10 years vs. TAM 5 years | Continue TAM to 10 years reduced breast cancer recurrence and mortality. |
| aTTom [125] | 6953 | After TAM for 5 years, continue TAM for 5 years vs. control | Continue TAM to 10 years reduced recurrence after 10 years |
| MA.17 [120] | 5187 | After TAM for 5 years, continue TAM for 5 years vs. placebo for 5 years | Improved DFS overall and distant DFS and survival in the node-positive subgroup |
| DATA [116] | 1912 | After TAM for 2-3 years, continue ANA 3 years vs. 6 years | Improved DFS only in the high-risk subgroup |
| MA.17R [120] | 1918 | After 5 years of AI, continue LET for 5 years vs. placebo for 5 years | Improved DFS and reduced breast cancer recurrence and contralateral breast cancer occurrence. |
| NSABP B-42 [117] | 3966 | After 5 years of AI (or TAM followed by an AI), continue LET for 5 years vs. placebo for 5 years | Compared with placebo, 5 years LET did not significantly prolong 7-year DFS |
| IDEAL [119] | 1824 | After 5 years of any initial endocrine therapy, continue LET for 5 years vs. 2.5 years | No superiority in DFS, OS and DFI with 5 years over 2.5 years of extended adjuvant letrozole. |
| SOLE [121] | 4884 | After 4-6 years of initial endocrine therapy, followed by continuous use of letrozole vs. intermittent use of letrozole (both for 5 years) | No significant difference in DFS in the intermittent letrozole group compared with the continuous letrozole group. |
- Abbreviations: TAM, tamoxifen; ANA, anastrozole; AI, artificial intelligence; LET, letrozole; DFS, disease-free survival; OS, overall survival; DFI, distant metastasis-free interval.
To optimize the ET strategy for premenopausal patients, two randomized trials (SOFT and TEXT) were conducted to test the effect of ovarian function suppression (OFS) [126-129]. Premenopausal women with hormone receptor (HR)-positive tumors were randomly assigned to receive tamoxifen, tamoxifen plus OFS, or exemestane plus OFS for 5 years in the SOFT trial receive 5 years exemestane plus OFS or tamoxifen plus OFS in TEXT after chemotherapy. In SOFT, the 8-year DFS rate was 78.9% in the tamoxifen alone group, 83.2% in the tamoxifen plus OFS group, and 85.9% in the exemestane plus OFS group (P = 0.009 for tamoxifen alone vs. tamoxifen plus OFS). The 8-year OS rate was 91.5% for tamoxifen alone, 93.3% for tamoxifen plus OFS, and 92.1% for exemestane plus OFS (P = 0.01 for tamoxifen alone vs. tamoxifen plus OFS). Analysis of recurrence risk showed that premenopausal women with HR-positive/HER2-negative disease at high risk, as evaluated by clinicopathologic characteristics, may get a 10% to 15% absolute improvement in 8-year freedom from distant recurrence with exemestane plus OFS vs. tamoxifen plus OFS or tamoxifen alone [130]. The 12-year follow-up results of the SOFT/TEXT continue to show that the use of OFS plus either tamoxifen or exemestane vs. tamoxifen alone for 5 years results in long-term reduction in distant recurrence risk, with the reduction being greatest in those who receive exemestane. The use of OFS also reduced the long-term risk of death. An EBCTCG meta-analysis aimed to compare the efficacy of OFS plus AI vs. OFS plus tamoxifen in premenopausal women with ER-positive early breast cancer by collecting data from 7030 women from four trials (ABCSG XII, SOFT, TEXT, and HOBOE). At a median follow-up of 8.0 years, the breast cancer recurrence rate was lower for women receiving an AI than for those receiving tamoxifen (RR = 0·79, 95% CI = 0·69-0·90, P = 0·0005). The main benefit was seen in the first 4 years, with a 3.2% absolute reduction in 5-year recurrence risk (6.9% vs. 10.1%) [131]. Based on these results, OFS, combined with an aromatase inhibitor or tamoxifen, should be considered for all premenopausal women with an indication for chemotherapy. Those with lower recurrence risk and without an indication for chemotherapy can be treated with tamoxifen alone because the potential benefit for escalating ET is minimal [130].
The exciting results observed in clinical trials of CDK4/6 inhibitors in the treatment of advanced ER-positive, HER2-negative breast cancer have led to exploring these agents in the adjuvant setting. MonarchE is a phase III, open-label trial that randomized 5637 patients to receive abemaciclib plus ET or ET alone. Abemaciclib was applied at 150 mg orally twice daily for 2 years. Patients with high-risk factors were enrolled in cohort 1 (≥4 positive ALNs, or 1-3 positive ALNs and either grade 3 disease or tumor ≥5 cm) or cohort 2 (1-3 positive ALNs and Ki-67 index ≥20%). The primary endpoint was invasive disease-free survival (IDFS) in the ITT population (cohorts 1 and 2). At a median follow-up of 27 months, 90% of patients were off treatment. Significant benefit was observed for both IDFS (hazard ratio = 0.70, 95% CI = 0.59-0.82; nominal P < 0.0001) and DRFS (hazard ratio = 0.69, 95% CI = 0.57-0.83; nominal P < 0.0001) in the abemaciclib plus ET treated group [132]. The absolute improvements in 3-year IDFS and DRFS rates were 5.4% and 4.2%, respectively. Findings from the MonarchE data have led to the U.S. Food and Drug Administration (FDA) approval of abemaciclib in adjuvant therapy of HR-positive and HER2-negative early breast cancer with a high risk of recurrence and Ki67 ≥20% [133]. Comparable study design exists for palbocicilb (PALLAS trial) and ribociclib (EarLEE-1 trial and NataLEE trial). The PALLAS trial evaluated the addition of 2 years of the CDK4/6 inhibitor palbociclib to standard adjuvant ET in patients with stage II-III breast cancer. The 3-year invasive DFS was 88·2% for palbociclib plus ET and 88.5% for ET alone (hazard ratio = 0.93 [95% CI = 0.76-1.15]; log-rank P = 0.51) [134]. Despite high discontinuation rates in PALLAS (42.2%), it was found that the lack of significant IDFS difference was not directly associated with inadequate palbociclib exposure [135]. The inconsistent results between MonarchE and PALLAS might be due to the disparity in the proportion of patients at high risk. Only 59.7% of participants in the PALLAS study met the inclusion criteria of cohort 1 in MonarchE (ALN+ ≥4 or 1≤ ALN+ ≤3 with either grade 3 or tumor ≥5 cm). Also, in the double-blind, phase III trial PENELOPE-B (NCT01864746), adding 1-year palbociclib treatment to ET did not improve the IDFS of women with HR-positive, HER2-negative primary breast cancer without pCR after taxane-containing NACT and at high risk of relapse (clinical pathological staging-ER grading score ≥ 3 or 2 and ypN+) [136]. Most enrolled patients in the PENELOPE-B trial were ypN+ with Ki-67 ≤ 15%. The role of CDK4/6 inhibitors in the adjuvant treatment of breast cancer needs further exploration, as well as the optimal treatment duration and patient selection.
Neoadjuvant ET (NET) has a role in older patients with ER-positive breast cancer who are inoperable or not able or unwilling to receive chemotherapy. In postmenopausal women, AIs are better than tamoxifen in the neoadjuvant setting, with response rates of ∼60% and an increase in breast conservation rate [137, 138]. NET should be given for 4-6 months. Although accepted as a surrogate marker for the long-term outcome of neoadjuvant chemotherapy, pathological complete response is not feasible for assessing NET. Whereas NET for 2-4 weeks might be used to predict the efficacy of endocrine treatment, as indicated by therapy-induced changes in Ki-67 [139]. The Preoperative Endocrine Prognostic Index (PEPI), a score based on post NET ER, Ki-67, tumor size, and nodal status assessed in the surgical specimen, is also predictive of the risk of relapse [139, 140]. Recent studies revealed that the effect of decreased proliferation after NET could be increased by adding a CDK4/6 inhibitor [141]. More phase III trials are ongoing to assess the role of a CDK4/6 inhibitor in neoadjuvant settings.
7.2 Chemotherapy for ER-positive and HER2-negative breast cancer
For selected patients, systemic adjuvant or neoadjuvant chemotherapy is administrated to reduce the risk of cancer recurrence. The decision to use chemotherapy should be based on considering and balancing the risk of recurrence, the absolute benefit of chemotherapy and the toxicity of therapy. The aim of adjuvant chemotherapy is to eradicate undiscovered distant metastases and improve patients’ long-term survival. The preferred adjuvant or neoadjuvant chemotherapy regimen includes sequential taxane-based regimen with or without anthracyclines [142]. The use of anthracyclines is controversial and could be considered for patients with a high risk of recurrence [143, 144]. Dose-dense regimens showed survival benefits compared with conventional dosed regimens [145, 146]. To identify candidates for adjuvant chemotherapy, clinical and pathological characteristics are taken into consideration to stratify patients’ risk of recurrence, including tumor grade or proliferation index, tumor size, nodal involvement, lymphovascular invasion, HR status and HER2 expression [147]. In cases of uncertainty, gene expression assays, such as MammaPrint, Oncotype DX, Prosigna, Endopredict or BCI, may be used when indicated. Analyses of the TAILORx, RxPonder, MINDACT and related studies have demonstrated that these assays can aid in determining an individual's risk of recurrence and potentially predict benefits derived from adjuvant chemotherapy [148-150]. Based on results from these studies, postmenopausal patients with invasive ductal or lobular tumors and 0 to 3 lymph node involvement had lower risk genomic signatures (defined as a recurrence score less than 25 on the 21-gene assay, or ‘low risk’ result on the 70-gene assay), there was no indication to routinely use adjuvant chemotherapy, while chemotherapy was recommended in patients with high genomic risk scores. For premenopausal patients with lower-risk genomic signatures, subset analyses from each of these trials indicated substantial benefit derived from chemotherapy, although some believe that the benefit might be due to ovarian suppression induced by chemotherapy. Considering the convergent results from the studies, adjuvant chemotherapy may not be routinely omitted in premenopausal patients with one to three positive axillary nodes and recurrence scores under 25 or other lower-risk genomic signatures.
7.3 Chemotherapy in patients with TNBC
Generally, neoadjuvant therapy is preferred for stage II or III tumors of triple-negative or HER2-positive subtypes. For stage II and stage III triple-negative cancers, dose-dense anthracycline and taxane-based chemotherapy are the standard of care. An anthracycline-free regimen may be considered for stage I triple-negative cancer patients [143, 144]. Several studies have explored the role of platinum agents in the treatment of patients with triple-negative disease in neoadjuvant settings [151-154], which showed improved pCR rates with the addition of platinum. Recently, Poggio et al. [155] reported an updated meta-analysis of RCTs assessing the survival benefit associated with platinum-based neoadjuvant chemotherapy in TNBC. The study found that compared with non-platinum neoadjuvant regimens, platinum-based chemotherapy significantly increased event-free survival (EFS; hazard ratio = 0.70, 95% CI = 0.56-0.89), and a non-significant 18% reduction in the risk of death (hazard ratio = 0.82, 95% CI = 0.64-1.04) was observed. Based on the result of EFS improvement, a platinum agent could be considered adding to the anthracycline- and taxane-based regimen as neoadjuvant chemotherapy backbone in early TNBC. In the adjuvant setting, the PATTERN trial showed improved DFS in early-stage triple-negative patients with adjuvant paclitaxel-plus-carboplatin versus epirubicin, fluorouracil, and cyclophosphamide followed by docetaxel, although the analysis was underpowered regarding the association of efficacy and HRR variation or BRCA1/2 status [156]. However, the PATTERN trial has some difficulties in its interpretation—it's not a classic design of adding a new drug to the same backbone; no dose-dense regimen used in either arm; the carboplatin arm also received weekly paclitaxel and not docetaxel, while the ECOG E1199 trial has shown that weekly paclitaxel may be the best taxane for early TNBC [157]. Other studies investigated the role of capecitabine in the treatment of early TNBCs [67, 158–160]. The randomized SYSUCC-001 trial evaluated the efficacy of capecitabine (650 mg/m2 twice a day for 1 year without interruption) maintenance after standard adjuvant chemotherapy in early-stage TNBC (T1/T2 stage, 93.1%; node-negative, 61.8%) [161]. Analysis of the study showed that low-dose capecitabine maintenance therapy, compared with observation, was associated with significantly higher 5-year DFS (82.8% vs. 73.0%; hazard ratio = 0.64). The SYSUCC-001 trial offered a new adjuvant treatment recommendation to help patients with TNBC achieve better long-term outcomes. For patients with high-risk, HER2-negative early breast cancer and germline BRCA1/2 mutations, adjuvant olaparib for 1 year after standard neoadjuvant or adjuvant chemotherapy significantly improved 3-year invasive DFS versus placebo (85.9% vs. 77.1%; hazard ratio = 0.58), as revealed by the OlympiA study [44].
In the neoadjuvant setting, adding programmed cell death protein 1 (PD-1) antibody pembrolizumab to paclitaxel and carboplatin followed by doxorubicin-cyclophosphamide significantly increased pCR rate with an absolute difference of 13.6% (64.8% vs. 51.2%, P = 0.00055), as shown by the phase III KEYNOTE-522 study at the primary analysis for pCR [54]. At the median follow-up of 39.1 months, the estimated EFS was significantly improved in the pembrolizumab-chemotherapy group (hazard ratio = 0.63; 95% CI = 0.48 to 0.82) [162]. Intriguingly, the pCR data from all 1174 patients at the third interim analysis showed a smaller absolute difference between arms compared to the first analysis (63% vs. 55.6%), indicating that pCR difference can translate into EFS benefit with the application of immunotherapy pre- and post- surgery [163]. Similar results were observed in the IMpassion 031 trial, which showed the addition of atezolizumab to nab-paclitaxel followed by doxorubicin-cyclophosphamide increased pCR in early TNBC [164]. In both trials, immune checkpoint inhibitors were given for a total of 1 year before and after surgery. These results might have changed the treatment strategies for patients with early TNBC. However, there are questions that remain to be determined, such as, is there an optimal biomarker that could identify candidates who benefit from neoadjuvant checkpoint inhibitor therapy? Subgroup analysis of KEYNOTE-522 did not reveal any biomarker that can predict the benefit of pembrolizumab, including PD-L1 expression, although it has been established as a predictive biomarker in the advanced setting. Novel biomarkers that have shown promising data in selecting patients for immunotherapy, such as circulating tumor DNA (ctDNA), TILs and expression of MHC-II complex, warrant further study in this field. Another important question is that is pCR a good enough predictor of EFS benefit in patients receiving neoadjuvant checkpoint inhibitors? Indeed, in the KEYNOTE-522, the major absolute benefit in terms of EFS was observed in the non-pCR population, with only 2% improvement in 3-year EFS for patients receiving pembrolizumab in those achieving pCR. This finding, together with results from GeparNuevo [165], showing improved survival outcomes with durvalumab added to neoadjuvant chemotherapy despite a small pCR increase and immunotherapy not continued after surgery, raised the possible strategy of de-escalating post-surgical immunotherapy in patients who achieved pCR, which needs to be examined in prospective trials. Moreover, what is the optimal adjuvant therapy for non-pCR patients after neoadjuvant immunotherapy? How to integrate immunotherapy into the current arsenal of treatment options? First, the CREATE-X trial demonstrated that the addition of 6-8 cycles of capecitabine to standard treatment improved DFS and OS in TNBC patients not achieving pCR after neoadjuvant treatment. Recently, addition of olaparib to standard treatment for one year in BRCA1- or BRCA2-mutated TNBC patients not achieving pCR after neoadjuvant therapy showed improvement in PFS and a trend towards improved OS in the OlympiA study. While all patients in the study group of KEYNOTE-522 received pembrolizumab as adjuvant treatment regardless of pCR status, neither capecitabine nor olaparib was allowed in this trial. Nonetheless, to derive maximum benefit from available treatments in clinical practice, adjuvant therapy in non-pCR patients may be tailored based on overall recurrence risk, residual disease burden and BRCA status. For TNBC patients with the highest risk of recurrence, it is reasonable to add adjuvant capecitabine to pembrolizumab or consider adjuvant olaparib ± pembrolizumab in BRCA mutation carriers, for both regimens have available data supporting the safety of the combination regimen.
7.4 Targeted therapy for early HER2-positive breast cancer
HER2 targeted therapy has changed the natural history of HER2-positive breast tumors. Most patients with a tumor larger than 2 cm or at least one positive lymph node should receive adjuvant or neoadjuvant trastuzumab and pertuzumab in addition to taxane-based chemotherapy with or without anthracyclines. Neoadjuvant docetaxel plus dual HER2 blockade with trastuzumab and pertuzumab resulted in a pCR rate of 65%-70% and improved EFS and DFS, suggesting the safety of anthracycline-free regimens in the era of potent anti-HER2 therapy [166, 167]. In the adjuvant setting, adding pertuzumab to trastuzumab and chemotherapy significantly improved the IDFS rate in patients with HER2-positive, operable breast cancer, especially for patients with node-positive disease [166]. For patients with low-risk, HER2-positive stage I tumors, paclitaxel plus trastuzumab has been generally accepted as a standard of care. This is based on a non-randomized APT trial (NCT00542451), showing that the 7-year rate of DFS was 93% (95% CI = 90%-93%) for patients receiving weekly paclitaxel for 12 weeks and trastuzumab for 1 year [168, 169]. In high-risk HER2-positive breast cancer, an additional adjuvant treatment of neratinib for 1 year after completion of trastuzumab can improve IDFS, as demonstrated by the ExteNET study [170, 171]. This study also showed that addition of neratinib might decrease the risk of central nervous system recurrence.
8 MBC
8.1 Endocrine-based therapy for HR-positive, HER2-negative MBC
ET is the preferred treatment for HR-positive, HER2-negative MBCs, unless immediate responses need to be achieved in rapid progressing and symptomatic diseases, in which cases chemotherapy may be considered. Patients have multiple hormonal therapy options, combined with increasing targeted therapy options, with improving clinical outcomes. Based on the evidence of multiple randomized trials of CDK4/6 inhibitors showing substantial improvements in response rate, progression-free survival (PFS) and in some circumstances OS, and the overall tolerability of CDK4/6 inhibitors, patients should receive ET plus a CDK4/6 inhibitor as the standard of care [172-178]. CDK4/6 inhibitors can be combined with an AI (preferably for treatment-naive or endocrine-sensitive MBC) or fulvestrant or tamoxifen in first, second or further lines and in postmenopausal and premenopausal patients.
Alpelisib, a PI3Kα-specific inhibitor, in combination with fulvestrant, can be offered to PIK3CA-mutated patients following prior ET, including an AI, with or without a CDK4/6 inhibitor. In the randomized, phase III SOLAR-1 trial, adding alpelisib to fulvestrant yielded significant improvement in PFS and a trend for improved OS in patients with PIK3CA-mutant tumors (in exons 9 or 20, detected in tumor tissue or circulating tumor DNA) [57, 179]. Another option is the mTOR inhibitor everolimus, that combined with exemestane, to be offered to patients with HR-positive, HER2-negative MBC who experience progression during or after treatment with non-steroidal AIs, either before or after treatment with fulvestrant, because PFS but not OS was significantly improved compared with exemestane alone [180, 181]. Single-agent abemaciclib is also an option for those with disease progressed on prior ET and multiple systemic therapies in the metastatic setting [182]. Histone deacetylase (HDAC) inhibitors represent another option for HR-positive advanced breast cancer patients who had progressed after prior ET [183]. In a phase III study, Chinese advanced breast cancer patients (n = 354) were randomized 2:1 to receive entinostat at 5 mg weekly plus exemestane at 25 mg daily (n = 235) or placebo plus exemestane. Results showed that the median PFS was 6.32 months (95% CI = 5.30-9.11) with the addition of entinostat verses 3.72 months (95% CI = 1.91-5.49) with placebo/exemestane (hazard ratio = 0.74; 95% CI = 0.58-0.96; P = 0.021) [184]. The OS data were not mature yet and were expected to be released in 2022.
The optimal sequence for ET in HR-positive, HER2-negative MBC is not fully defined. In the SOLAR-1 trial, only 5.9% of patients in the PIK3CA-mutated cohort had received prior CDK4/6 inhibitor therapy. Additional data on outcomes of alpelisib in the post CDK4/6 inhibitor setting are available from the non-randomized BYLieve trial [185]. Results of cohort A of the study showed that patients with PIK3CA-mutated diseases receiving alpelisib and fulvestrant after progression with a CDK4/6 inhibitor plus an AI as the immediate prior therapy had a median PFS of 7.3 months and 50.4% (61 of 121) were alive without disease progression at 6 months. Cohort B of the study enrolled patients who had to have progressed on a CDK4/6 inhibitor and fulvestrant as their last treatment. With a median follow-up of 15 months, alpelisib plus letrozole resulted in a median PFS of 5.7 months (n = 126; 95% CI = 4.5-7.2). Moreover, 46.1% of patients were alive without disease progression at 6 months (n = 53; 95% CI = 36.8%-55.6%), meeting the study's primary end point. Cohort C of the study enrolled patients who progressed on or after treatment with an aromatase inhibitor and received chemotherapy or ET as immediate prior treatment. A total of 67.5% of the cohort C patients also received a CDK4/6 inhibitor as prior treatment. Patients enrolled in cohort C were treated with alpelisib plus fulvestrant. At the 6-month assessment, 48.7% were alive without disease progression and the median PFS was 5.6 months [186]. These results support the sequential use of alpelisib after CDK4/6 inhibitors. There are also very limited data regarding the outcomes of everolimus after CDK4/6 inhibitors [187]. Based on efficacy and tolerability, most guidelines recommend CDK4/6 inhibitors in combination with ET to be used in the first-line setting. Alpelisib, everolimus or entinostat may be used as second or subsequent lines of endocrine-based therapy (Figure 2) [188].
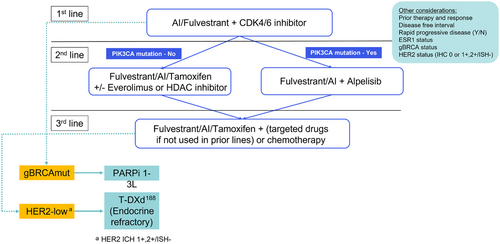
A phase III PARSIFAL trial (NCT02491983) aimed to identify the best endocrine partner of CDK4/6 inhibitors in the first-line scenario [189]. A total of 468 patients with ER-positive/HER2-negative MBC with no prior treatment in the advanced setting and endocrine-sensitive disease were randomized to receive palbociclib in combination with letrozole or fulvestrant. At median follow-up of 32 months, no significant difference in median PFS were observed between palbociclib plus fulvestrant group (27.9 months; 95% CI = 24.2-33.1) or letrozole group (32.8 months; 95% CI = 25.8-35.9; hazard ratio = 1.1; P = 0.321). No differences were found in 4-year OS rates, overall response rate (ORR) or clinical benefit rate (CBR) between the two arms as well. A pooled analysis by U.S. FDA included 4200 patients from seven phase 3 randomized studies of CDK4/6 inhibitors plus ET. Results of this study showed that PFS favored the CDK4/6 inhibitor plus group in all prespecified clinicopathological subgroups analyzed. Similar hazard ratio values were observed when pooling the PFS results by AI in the first-line setting (hazard ratio = 0.55; 95% CI = 0.49-0.62) or fulvestrant (hazard ratio = 0.58; 95% CI = 0.51-0.65), or in pooled analysis for fulvestrant in the first-line (hazard ratio = 0.58; 95% CI = 0.42-0.80) or second-line and beyond setting (hazard ratio = 0.56; 95% CI = 0.49-0.64) [190]. Based on these data, both fulvestrant and AIs are reasonable choices of endocrine partner for CDK4/6 inhibitors in the first-line treatment of patients with endocrine-sensitive MBC. On the other side, for patients with the endocrine-resistant disease, the enrichment of estrogen receptor 1 (ESR1) mutations in this population could impair the efficacy of the combination of CDK4/6 inhibitor and AI, as revealed by the PADA-1 trial [191]. In the phase III liquid biopsy-based trial, ER-positive HER2-negative MBC patients were treated with AI plus Palbociclib in the first line and tracked with ESR1 mutations in ctDNA by droplet digital PCR (ddPCR). Upon detecting ESR1 mutations in blood and without a clinical/imaging disease progression, patients were randomized to continue AI plus palbociclib or switch to fulvestrant plus palbociclib. Among the 172 patients subjected to randomization, the median PFS was 5.7 months in the AI-palbociclib arm and 11.9 months in the fulvestrant-palbociclib arm (hazard ratio = 0.63; 95% CI = 0.45-0.88], P = 0.007) after a median follow-up of 26 months. Among the 70 patients in the AI-palbociclib arm who subsequently progressed, 47 were crossed over to receive fulvestrant-palbociclib. With a median follow-up of 14.7 months, the median second PFS in the cross-over patients was 3.5 months. Considering the significantly improved PFS for fulvestrant plus palbociclib over AI plus Palbociclib in ctDNA ESR1mut patients and the limited PFS of the cross-over group after disease-progression, fulvestrant-palbociclib should be considered as an optimal choice for patients with known ESR1 mutations or AI-resistant diseases.
Although combination therapies appear to be beneficial to most patients, endocrine monotherapy remains a first-line therapy choice for those with limited disease burden, long disease-free intervals and old age. Considerations should also include patient choice and other factors such as treatment tolerance. In this situation, CDK4/6 inhibitors could be applied in combination with second-line ET.
8.2 Chemotherapy and targeted therapy for patients with HER2-negative MBC
For patients with metastatic PD-L1-positive triple-negative (HR-negative, HER2-negative) breast cancer, the addition of an immune checkpoint inhibitor to chemotherapy (atezolizumab plus nab-paclitaxel or pembrolizumab plus chemotherapy) could be offered as a first-line treatment. The KEYNOTE-355 trial investigated the efficacy of the checkpoint inhibitor pembrolizumab in combination with chemotherapy (nab-paclitaxel, paclitaxel, or gemcitabine/carboplatin) in 847 previously untreated metastatic TNBC patients [56]. The addition of pembrolizumab to chemotherapy resulted in a modest clinical benefit in the intent-to-treat population, but significantly improved PFS (9.7 months vs. 5.6 months; hazard ratio = 0.65, 95% CI = 0.49-0.86) and OS (23.0 months vs. 16.1 months; hazard ratio = 0.73, 95% CI = 0.55-0.95) were observed for the pembrolizumab-chemotherapy group in patients with PD-L1 combined positive score (CPS) ≥10%, as detected using the 22C3 antibody [15]. The IMpassion130 trial randomized 902 patients with metastatic TNBC who were not treated in the metastatic setting to receive the PD-L1 inhibitor, atezolizumab plus nab-paclitaxel or placebo plus nab-paclitaxel [55, 192, 193]. Analyses showed a modestly improved PFS with the addition of atezolizumab in the entire study population but indicated a significant PFS improvement (7.5 months vs. 5 months; hazard ratio = 0.62, 95% CI = 0.49-0.78) and a clinical meaningful OS benefit (25 months vs. 15.5 months; hazard ratio = 0.62, 95% CI = 0.45-0.86) in the PD-L1-positive subset of patients. In the IMpassion130 study, PD-L1-positivity was defined as stanning at any intensity in ≥1% tumor-infiltrating immune cells using the SP142 antibody. In contrast, the IMpassion131 trial, applying a study design similar to the IMpassion130 study, showed that the addition of atezolizumab to paclitaxel did not improve median PFS in the PD-L1-positive subgroup [194]. Therefore, the application of atezolizumab in metastatic TNBC is controversial. Also, when used in patients with metastatic TNBC, atezolizumab should be paired with nab-paclitaxel, not paclitaxel.
Sacituzumab govitecan is a trophoblast cell-surface antigen 2 (Trop-2) directed antibody conjugated to SN-38, an active metabolite of the topoisomerase I inhibitor irinotecan. The randomized phase III ASCENT trial allocated 529 patients with metastatic TNBC that failed two or more prior regimens to receive sacituzumab or single-agent chemotherapy of physician's choice (eribulin, vinorelbine capecitabine, or gemcitabine) [195]. Among the 468 without brain metastases, sacituzumab govitecan significantly improved both PFS (5.6 months vs. 1.7 months; hazard ratio = 0.41, 95% CI = 0.32-0.52) and OS (12.1 months vs. 6.7 months; hazard ratio = 0.48, 95% CI = 0.38-0.59) as compared with standard therapy. Results of the ASCENT trial support the use of sacituzumab govitecan for treating metastatic TNBC who have received at least two previous therapies for advanced disease.
About 5% of all breast cancers are associated with the BRCA1/2 germline mutations, with the majority of which being HER2-negative (HR-positive or HR-negative). Two randomized phase III clinical trials support the use of PARP inhibitors (olaparib or talazoparib) in patients with MBC with BRCA1/2 germline mutation [40, 196–198]. The OlympiAD trial randomly assigned 302 patients with metastatic HR-positive or TNBC to receive olaparib vs. single-agent chemotherapy of the physician's choice (vinorelbine, capecitabine, or eribulin). Median PFS was significantly better in the olaparib group than in the standard therapy group (7.0 months vs. 4.2 months; hazard ratio = 0.58, 95% CI = 0.43-0.80). The PFS benefit noted with Olaparib were more pronounced in the triple-negative subset of 102 patients. Similarly, the EMBRCA trial showed that talazoparib significantly improved PFS compared to standard therapy of physician's choice (8.6 months vs. 5.6 months; hazard ratio = 0.54, 95% CI = 0.41-0.71) in patients with advanced breast cancer carrying germline BRCA mutations. Neither of the two studies indicated significant OS benefit with PARP inhibitor therapy over standard therapy. The role of somatic BRCA1/2 mutations in breast cancer is still to be determined in further research and currently should not be considered as an indication for PARP inhibitors in routine practice. Considering the clinical efficacy and OS benefit, the optimal sequence in patients with PD-L1-positive and BRCA1/2 germline mutations would be checkpoint inhibitor-based therapy in the first and the PARP inhibitor in the latter [199].
Patients with metastatic TNBC without PD-L1 expression (PD-L1-negative) or BRCA1/2 germline mutation should be offered chemotherapy as first-line treatment. For patients with HR-positive, HER2-negative tumors associated with symptomatic visceral metastasis or refractory to ET, chemotherapy remains a choice. Sequential single-agent chemotherapy is preferred rather than combination chemotherapy, although combination regimens may provide a higher objective response rate and longer time to progression, and is useful for patients with rapid disease progression or in need of immediate symptom or disease control. Preferred single agent regimens for advanced MBC include taxanes, anthracyclines, anti-metabolites (capecitabine and gemcitabine), microtubule inhibitors (vinorelbine, eribulin, and utidelone), platinum agents for triple-negative tumors and germline BRCA1/2 mutation carriers. Eribulin mesilate is a non-taxane microtubule dynamics inhibitor. In a phase III EMBRACE trial, eribulin treatment (1.4 mg/m2 on day 1 and day 8 of a 21-day cycle) resulted in a significantly improved OS in heavily pretreated patients when compared with treatment of physician's choice [200]. In another phase III trial (NCT02225470), eribulin showed superiority in both PFS and tolerability compared with vinorelbine in previously treated recurrent or MBC [201]. Utidelone is a genetically engineered epothilone analogue, and its combination with capecitabine significant improved PFS (8.44 months vs. 4.27 months by central review, hazard ratio = 0·46, 95% CI = 0·36-0·59) and OS (19.8 months vs. 16.0 months, hazard ratio = 0.75, 95% CI = 0.59-0.94) as compared with capecitabine alone in heavily-pretreated, anthracycline- and taxane-resistant MBC patients, as demonstrated in a multi-center phase III study conducted in China [202, 203]. These results support using utidelone as a novel cytotoxic agent in patients with MBC pretreated with anthracyclines and taxanes.
8.3 Management of HER2-positive metastatic breast cancer
For HER2-positive metastatic breast cancer, HER2-targeted therapy should be offered beyond progression since it is beneficial to continue HER2 pathway suppression. The recommended first-line therapy is currently dual HER2 blockade with trastuzumab and pertuzumab in combination with chemotherapy (preferably taxanes) [204, 205]. Patients with HR-positive and HER2-positive tumors have the alternative of receiving ET in combination with HER2-targeted therapy [206-208]. The second line options include trastuzumab deruxtecan (T-DXd), T-DM1, and pyrotinib (approved in China). Trastuzumab deruxtecan is a next-generation antibody-drug conjugate composed of an anti-HER2 antibody linked to a cytotoxic topoisomerase I inhibitor [209]. In the phase III DESTINY-Breast03 trial, T-DXd showed significant improvement in PFS versus T-DM1 (hazard ratio = 0.2840; P = 7.8 × 10−22) in second-line treatment for HER2-positive unresectable or MBC [210]. Another trial, DESTINY-Breast09, comparing T-DXd with pertuzumab and trastuzumab plus docetaxel, the current first-line standard-of-care regimen, is ongoing. A phase III randomized PHOEBE trial showed that the combination of capecitabine and pyrotinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor, significantly improved PFS compared with lapatinib plus capecitabine (12.5 months vs. 6.8 months; hazard ratio = 0.39, 95% CI = 0.27-0.56) in HER2-positive MBC after previous trastuzumab and taxanes [211]. As for the subsequent line therapies, neratinib plus capecitabine was superior to lapatinib combined with capecitabine [212]. The highly selective anti-HER2 tyrosine kinase inhibitor tucatinib plus capecitabine and trastuzumab resulted in significantly better PFS and OS as compared with placebo plus capecitabine and trastuzumab in the overall population and in patients with brain metastases who were pretreated with trastuzumab, pertuzumab, and T-DM1, as indicated by the HER2CLIMB study [213]. Pyrotinib and T-DXd also showed impressive efficacy in HER2-positive patients with brain metastases (Table 3) [209, 214-219]. The development of novel anti-HER2 drugs will expand the arsenal for treating HER2-positive breast cancer patients, for whom continuous anti-HER2 treatment is essential.
| Clinical trial | Status | Treatment arms | Patients with BM | ORR (%) | mPFS (month) | mOS (month) |
|---|---|---|---|---|---|---|
| HER2CLIMB [213] | Has resultsF | All patients with BM | ||||
| Tucatinib/Trastuzumab/Cape | 198 | 47.3* | 9.9 | 21.6 | ||
| Trastuzumab/Cape | 93 | 20.0* | 4.2 | 12.5 | ||
| Has results | Active BM | |||||
| Tucatinib/Trastuzumab/Cape | 118 | NR | 9.6 | 21.4 | ||
| Trastuzumab/Cape | 56 | NR | 4.0 | 11.8 | ||
| Has results | Stable BM | |||||
| Tucatinib/Trastuzumab/Cape | 80 | NR | 13.9 | 21.6 | ||
| Trastuzumab/Cape | 37 | NR | 5.6 | 16.4 | ||
| NALA [212] | Has results | Neratinib/Cape | 51 | 28.6 | 5.6 | 13.9 |
| Lapatinib/Cape | 50 | 28.2 | 4.3 | 12.4 | ||
| TBCRC 022 [218] | Has results | Neratinib/Cape (lapatinib naive) | 37 | 49* | 5.5 | 13.3 |
| Neratinib/Cape (prior lapatinib) | 12 | 33* | 3.1 | 15.1 | ||
| PATRICIA [219] | Has results | Pertuzumab + High-dose Trastuzumab | 39 | 11* | NR | NR |
| PERMEATE [214] | Has results | Pyrotinib/Cape (RT-naïve) | 51 | 74.6 | 11.3 | NR |
| Pyrotinib/Cape (RT-progressive) | 19 | 42.1 | 5.6 | NR | ||
| DESTINY Breast-01 [209] | Has results | T-DXd | 24 | 58.3 | 18.1 | NR |
| DESTINY Breast-03 [210] | Has results | T-DXd | 43 | 67.4 | 15.0 | NR |
| T-DM1 | 39 | 20.5 | 3.0 | NR | ||
| KAMILLA [216] | Has results | T-DM1 | 126 | 21.4 | 5.5 | 18.9 |
| NCT04334330 | Ongoing | Palbociclib + pyrotinib + trastuzumab + fulvestrant | N/A | N/A | N/A | N/A |
| NCT03765983 | Ongoing | GDC-0084 (PI3K inhibitor) + trastuzumab | N/A | N/A | N/A | N/A |
| NCT03975647 | Ongoing |
Tucatinib + T-DM1 Placebo + T-DM1 |
N/A | N/A | N/A | N/A |
- Abbreviations: BM, brain metastasis; NR, not reported; N/A, not applicable; ORR, overall response rate; mPFS, median progression free survival; mOS, median overall sruvival; RT,radiotherapy; T-DXd, trastuzumab deruxtecan; T-DM1, trastuzumab emtansine.
- * CNS ORR
9 CONCLUSION AND PERSPECTIVE
In the recent 20 years, we have seen the development of personalized/precision treatment in breast cancer. Current precision treatment strategies are based on molecular subtyping of breast cancer. Future therapeutic concepts will focus more on individualization of therapy for every single patient and escalation and de-escalation of treatment according to tumor biology and early predictive markers. Further classification of the current breast cancer subtypes (e.g., TNBC) combined with subtyping-based umbrella trials may help improve the disease outcome [220]. In addition, developing novel drugs for both early and advanced breast cancer remains an unmet need. The mechanisms underlying drug resistance and ways to overcome it are the main focus of ongoing research. Single-cell technologies will provide insight into tumor-microenvironment interactions and may help to uncover new treatment biomarkers and targets. As an example, a single-cell study found that the level of the CXCL13-positive T cell subset was predictive of responses to anti-PD-L1 therapies in TNBC [221].
The major trend in breast cancer surgery is de-escalation. Future surgical treatment will focus more on tumor biology, and the treatment plan will be more individualized. In the future, two major questions in breast cancer treatment remain to be answered. Can breast surgery be omitted in patients with a pCR after neoadjuvant therapy? And the other, can some patients be completely spared axillary surgery, both for the purpose of staging and treatment. These are important questions, but randomized controlled trials to study them can be difficult because of safety concerns, highlighting the importance of international cooperation. Careful consideration to balance the potential decrease of adverse events and increase in the risk of recurrence needs to be taken and discussed individually. It is essential that all de-escalation strategies or concepts should be tested within clinical trial settings.
DECLARATIONS
CONSENT FOR PUBLICATION
Not applicable.
AUTHOR CONTRIBUTIONS
All authors contributed actively to the manuscript and approved the final version.
ACKNOWLEDGMENTS
This study was supported by CAMS Innovation Fund for Medical Sciences (CIFMS, 2021-I2M-1-014) and CAMS Initiative for Innovative Medicine (CAMS-12M-1-010).
COMPETING INTERESTS
The authors declare that they have no competing interests.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.




