The application and challenges of brain-computer interfaces in the medical industry
Qi Chen and Sha Zhao are equally contributed to this work.
Abstract
Brain-computer interface (BCI) technology aims to create a connection pathway for exchanging information between the brain and devices with computing capabilities. This technology has become a global research focus, and many countries and regions are working to establish a BCI industry. BCIs have many potential applications, especially in the medical field. However, the complexities of non-invasive BCIs and the implantation risks associated with invasive BCIs have limited these technologies to laboratory settings. The main challenges for the practical implementation of BCIs include the lack of foundational technologies for non-invasive and invasive BCIs, the signal processing challenges associated with BCIs, the key components of BCIs, and the compatibility of BCI software and hardware. These shortcomings should be addressed to enhance the competitiveness of BCI products and promote the application of BCIs in medicine. In the future, if novel methods for acquiring or decoding neural signals are developed that enable non-invasive BCIs to achieve signal quality comparable to that of invasive techniques, it will propel BCI technology to leapfrog in development. Technological breakthroughs will enable BCIs to enhance medical technology and improve people's quality of life.
Key Points
What is already known about this topic?
-
Brain-computer interfaces (BCIs) aim to establish a direct information pathway between the brain and external devices, making it possible to read brain information and input external information.
-
BCIs are becoming increasingly widespread.
What does this study add?
-
The foundational technologies for non-invasive and invasive BCIs, the signal processing challenges associated with BCIs, the key components of BCIs, and the compatibility of BCI software and hardware are the key challenges to be solved.
-
In the future, if novel methods for acquiring or decoding neural signals are developed that enable non-invasive BCIs to achieve signal quality comparable to that of invasive techniques, it will propel BCI technology to leapfrog in development.
1 INTRODUCTION
Brain-computer interfaces (BCIs) are novel communication and control methods1 that aim to establish a direct information pathway between the brain and external devices,2, 3 making it possible to read brain information and input external information (Figure 1). The core goal of the BCI is to replace, restore, or enhance brain function, for example, by assisting individuals with paralysis in controlling mechanical limbs and helping those with aphasia to communicate through speech synthesis. The BCI technology involves three steps: signal acquisition (electrode acquisition of brainwaves), signal decoding (the analysis of intent by an AI algorithm), and instruction execution (control of external devices).4-6 This technology integrates multiple disciplines, including artificial intelligence, neuroscience, microelectronics, materials science, and biomedical engineering. BCIs have become a global science and technology frontier, and many countries and regions are accelerating the layout of their BCI industries, particularly in the auxiliary diagnosis and treatment of patients.
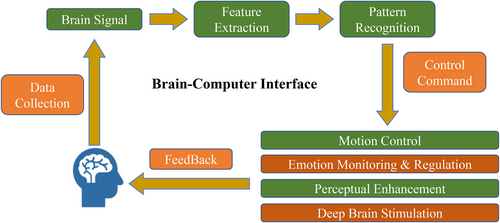
Brain-computer interface (BCI) diagram.
In laboratory settings, BCIs have been preliminarily used for brain-controlled robotic arms, writing, and speech systems, providing channels for patients to interact with the external world.7-9 Additionally, artificial cochleae and retinae constructed using BCIs have been used to restore sensory perception in patients with auditory and visual impairments.10 Closed-loop neural stimulators have recently been employed for treatment-resistant severe depression, improving therapeutic outcomes. Thus, BCIs have wide application potential in the medical field, particularly in the auxiliary diagnosis and treatment of neurological and psychiatric disorders. However, the complexities of non-invasive BCIs and the implantation risks associated with invasive BCIs have relegated these devices to laboratory settings. This study will discuss the key technologies, clinical application and medical industrialization of BCIs, and BCI companies. Furthermore, an outlook on future advancements and challenges is presented.
2 KEY BCI TECHNOLOGY
The core technology of the BCIs is in the precise decoding of neural activity, and their performance depends on the choice of signal acquisition technology.6 BCIs are divided into non-invasive and invasive BCIs based on the location and degree of signal acquisition. Non-invasive BCIs are a safer and more accessible options; thus, they can be used in research and industry. However, challenges such as signal attenuation and a low signal-to-noise ratio remain; nonetheless, each technical route has unique advantages, challenges, and application scenarios.
2.1 Non-invasive BCI technology
Non-invasive BCIs collect physiological signals related to neural activity from the scalp surface or in vitro without the need for surgical implantation.11 Non-invasive BCIs are safe, easy to deploy, and suitable for a wider range of subjects and daily application scenarios. However, non-invasive BCIs face challenges such as the low spatial resolution of signals and a low signal-to-noise ratio.
2.1.1 Electroencephalography (EEG)
EEG records the subtle potential changes generated by the synchronous activity of neurons in the cerebral cortex through scalp electrodes.12 EEG is the most extensively researched and widely used non-invasive BCI technology.1, 13 Its advantages include low cost, outstanding temporal resolution (in milliseconds), and portability. However, there are several drawbacks, including poor spatial resolution due to the susceptibility of EEG to the volume conductor effect and skull attenuation, susceptibility to signal interference from artifacts such as electromyography (EMG) and electrooculography (EOG), the need for conductive paste (wet electrode), and difficulty in maintaining adequate contact for long periods (dry electrode). The mainstream paradigm of EEG-BCI is a BCI based on features such as sensorimotor rhythm, event-related potential, and steady-state visual evoked potential.
2.1.2 Functional magnetic resonance imaging (fMRI)
fMRI indirectly reflects brain activity by detecting local hemodynamic changes (i.e., blood oxygen level-dependent signals) induced by neuronal activity.14 The greatest advantage of fMRI is its outstanding spatial resolution (in millimeters) under non-invasive conditions, enabling the generation of a whole-brain functional activity map. fMRI is useful for studying advanced brain functions, such as cognition and emotion, and for applications in BCIs for neurofeedback and consciousness detection.15 However, fMRI requires expensive and bulky equipment, has a low temporal resolution (in seconds), is extremely sensitive to head movements and high scanning noise, and cannot be used in a natural state, limiting its practicality as a real-time BCI control interface. Thus, fMRI is generally used in basic research and offline decoding.
2.1.3 Functional near-infrared spectroscopy (fNIRS)
fNIRS uses near-infrared light to penetrate the scalp and skull, indirectly reflecting the neural activity (hemodynamic response) of the subcortical brain region by measuring changes in the concentrations of oxygenated hemoglobin (HbO) and deoxyhemoglobin (HbR). The advantages of fNIRS include portability, higher resistance to motion interference in comparison with EEG, lower cost than fMRI, and a tolerance for a certain degree of hair occlusion. The spatial resolution of fNIRS is greater than that of EEG but lower than that of fMRI, and its temporal resolution (in seconds) is greater than that of fMRI but considerably lower than that of EEG. fNIRS-BCI is particularly suitable for decoding motor imagery and applications that require certain spatial information but do not have extremely demanding real-time requirements, such as neural rehabilitation training and mental state monitoring.16, 17
2.1.4 Magnetoencephalography (MEG)
MEG measures the weak extracranial magnetic field generated by neuronal electrical activity via a superconducting quantum interference device array.18, 19 The advantages of MEG include an extremely high temporal resolution (in milliseconds) and adequate spatial resolution, especially on the surface of the cortex. Furthermore, due to the more accurate spatial localization versus that achieved with EEG, the signal is not affected by the uneven conductivity of skull and scalp tissues. However, MEG equipment is expensive and bulky (requiring a magnetic shielding chamber and liquid helium cooling), has strict requirements for head movement, and the signal strength rapidly decays with distance; thus, it is difficult to detect signals in deep brain regions. These drawbacks hinder the application of MEG in practical and mobile BCIs;20 thus, MEG is more commonly used in cognitive neuroscience research21 and high-precision brain source localization.22
2.2 Invasive BCI technology
Invasive BCIs involve the surgical implantation of electrodes or sensors directly into intracranial tissues (such as the surface, cortex, or deep brain regions) to obtain neurophysiological signals with high spatiotemporal resolution.
2.2.1 Electrocorticography (ECoG)
ECoG was previously the most popular invasive BCI technique.23, 24 The ECoG electrode grid is usually placed under the dura mater and is closely attached to the surface of the cerebral cortex. Unlike non-invasive EEG, ECoG signals avoid the attenuation and spatial blurring effects of skull and scalp signals,25 with a higher signal-to-noise ratio, better spatial resolution (millimeter level), and a wider frequency band, effectively capturing high gamma frequency band activity (>70 Hz). Thus, ECoG is adequate for motion imagination decoding, language decoding, and high-precision control tasks. ECoG is widely used in invasive BCI clinical applications,23, 24, 26 such as epilepsy lesion localization, closed-loop neural regulation, multidegree-of-freedom prosthetics, and cursor control.
2.2.2 Stereotactic EEG/intracranial EEG (SEEG/iEEG)
SEEG/iEEG27, 28 is minimally invasive and is safer than ECoG. SEEG implants deep electrodes containing multiple contact points into the target brain area through minimally invasive drilling.28, 29 Its unique advantage is its ability to record the electrical activity of deep brain structures, such as the hippocampus, amygdala, and insula, which are crucial for studying complex cognitive functions, locating epileptic foci in drug-resistant epilepsy, and developing BCI applications targeting deep brain regions. However, SEEG has a low detection range for cortical cognitive brain regions.
2.2.3 Microelectrode arrays (MEAs) and single-unit activity (SUA)
MEAs30 and SUA31 are sophisticated invasive BCI technologies that involve the insertion of microelectrodes into cortical tissue to record the action potentials (spikes) of individual neurons or local small groups of neurons. SUA provides the most refined neural coding information, theoretically enabling the most direct and precise control of motion.32 However, the main challenges of SUA faces include the poor stability of long-term records due to signal attenuation caused by glial cell encapsulation, biocompatibility issues, risk of tissue damage, and physical limitations on the number of signal channels. Conversely, local field potentials (LFPs), as the sum of the low-frequency LFPs recorded by MEA, provide an integrated view of neuronal cluster activity with increased stability33; thus, LFPs are practical signal sources for many invasive BCI systems.
Endovascular/perivascular electrodes are interventional BCI technologies that deploy electrode scaffolds in veins near the cortex through vascular intervention surgery. This minimally invasive or semi-invasive method avoids the need for craniotomy, balancing signal quality (resembling ECoG levels) with safety (reducing tissue damage and infection risk). However, this technology is hindered by the unstable contact between electrodes and blood vessel walls, a lack of long-term signal stability, and a limited number of deployable locations and channels.
2.3 The advantages of non-invasive and invasive BCIs
The advantages of non-invasive BCIs include safety, ease of use, and wide user acceptance. Thus, non-invasive BCIs have substantial potential in basic research, neurofeedback rehabilitation, gaming and entertainment, auxiliary communication, mental state monitoring, and other fields. The main challenges associated with non-invasive BCIs are a low signal-to-noise ratio, low spatial resolution (in the case of EEG and fNIRS), susceptibility to artifact interference (EEG), and the bulky, expensive, and poor real-time performance of some technologies (e.g., fMRI and MEG). Electrode/sensor technology (e.g., high-density dry electrodes, comfort materials, and new optical sensors) and advanced signal processing algorithms (e.g., deep learning) are key research directions for improving the performance of non-invasive BCIs.
On the other hand, invasive BCIs achieve excellent signal quality (a high signal-to-noise ratio, high spatiotemporal resolution, and wide bandwidth), which provides a physical basis for decoding complex neural intentions and achieving precise and fast control. However, invasive BCIs face challenges including surgical implantation risks (e.g., infection, bleeding, and tissue damage), biocompatibility issues with long-term implantation (e.g., chronic inflammatory response and signal attenuation caused by glial scar formation), a lack of long-term stability, complex system packaging and wireless transmission, and strict regulatory requirements. Table 1 presents a comparison of the technical specifications of invasive and non-invasive BCIs.
| Type | Product/technology | Function | Target | Operational duration | Key parameters |
|---|---|---|---|---|---|
| Non-invasive BCI | Kernel flow | Using TD-fNIRS for monitoring neural activity | Human | Short-term (hours) | Equipped with 52 modules in a helmet form factor to cover the whole head |
| Cognixion ONE BCI (AR glasses) | Communication aid for ALS/cerebral palsy via a brain-controlled AR interface | Human | Real-time sessions | Combines AR technology with BCI; designed for accessibility and communication assistance | |
| Invasive BCI | Neuralink N1 implant | Motor control for paralysis | Human | Long-term (>1 year) | 1024 electrodes; wireless data transmission |
| Stentrode | Collects and decodes task-related neural signals | Human | - | Expandable mesh stent electrode; wireless signal transmission to external devices | |
| Percept PC | Deep brain stimulation for Parkinson's disease | Human | Long-term (>5 years) | BrainSense technology; MRI compatibility; battery longevity | |
| Paradromics Connexus DDI | High-bandwidth data interface for restoring communication in paralysis | Human (initial studies in animal models) | Long-term | Supports up to 65,536 electrodes; micro-implantable chip for high-data-rate communication |
3 BCIs IN THE MEDICAL INDUSTRY
3.1 Industry of non-invasive BCIs
The clinical application of BCIs based on EEG is currently the most widely utilized and mature of the non-invasive method. Non-invasive BCIs are broadly applied in areas such as motor rehabilitation, communication for locked-in patients, disease monitoring, and neural regulation.34
3.1.1 Clinical application based on EEG
In terms of rehabilitation and neuromodulation, the Austrian company g.tec demonstrated early possibilities by enabling an individual with paralysis to send messages on Twitter via a BCI system.35 More recently, Cognixion launched the Cognixion ONE, a wearable device combining an augmented reality display with a BCI, designed to assist patients with severe motor impairments like cerebral palsy and amyotrophic lateral sclerosis (ALS) in communication.36 In China, Borui Kang has developed medical-grade EEG diagnostic equipment. Zhentai Intelligent integrates virtual reality and BCIs to produce upper and lower limb rehabilitation robots. South China Brain Control created a brain-controlled wheelchair and an intelligent ward system.
Epilepsy prediction is a crucial application direction for monitoring and managing brain disease. The Minder device from the Australian company Epiminder has received Breakthrough Device designation from the U.S. FDA for its potential in monitoring and forecasting seizures. The Spanish company MJN Neuroserveis has developed a CE-certified headphone-style EEG device that uses AI algorithms to provide a warning before an epileptic seizure occurs. For mental health, Taiwan's Hongzhi Biomedical (Hondee Bio-Medical) has developed an eight-channel EEG system intended for the rapid diagnosis of depression, reporting an accuracy rate of over 80% in clinical trials.
In the field of sleep and emotion management, the Dreem headband (an FDA Class II medical device), developed in the United States, improves sleep quality through closed-loop auditory stimulation, although the company that produces it was later acquired. In China, Brainland Technology's (Naolu) SleepUp patch and the AirDream Flexible patch provide non-invasive sleep interventions.
Addressing neurodevelopmental disorders such as attention deficit hyperactivity disorder (ADHD) and autism is another key research direction. Neeuro (Singapore) developed an EEG-based attention training game. Similarly, BrainCo (QiangNao Technology) in China has created the “Kaixingguo” system, a BCI-based tool designed to provide social and cognitive training programs for children with autism.
3.1.2 Clinical application based on fNIRS
fNIRS technology has rapidly advanced for application in BCIs, especially in rehabilitation training and interventions for pediatric neurological diseases.37 This advancement results from fNIRS's ability to measure changes in cerebral cortex hemodynamics (oxygenated and deoxygenated hemoglobin concentration), its insensitivity to motion artifacts, and equipment portability. Compared with EEG, fNIRS provides greater spatial resolution and tissue-specific information. Consequently, fNIRS is widely used in BCIs for motor rehabilitation to decode motor imagery (e.g., hand and foot movements), drive exoskeletons, or functional electrical stimulation for post-stroke upper limb functional training. Studies have shown that fNIRS exhibits high stability and a smooth learning curve in patients with chronic stroke.38 Regarding pediatric applications, fNIRS has become one of the preferred tools for neurofeedback training in autism spectrum disorder (ASD)39 and ADHD40 due to its comfort and tolerance for head movements. In particular, fNIRS is used to train attention regulation, emotional control, and social cognitive abilities.
3.1.3 Clinical application based on fMRI
Although the use of fMRI for daily BCI control is impractical because of its bulky and expensive equipment and limited temporal resolution (on the scale of seconds), its excellent spatial resolution (in millimeters) and whole-brain coverage are advantageous for specific BCI applications. Thus, fMRI is one of the gold standards for the preoperative localization of key functional areas, such as movement and language, providing crucial anatomical and functional information for target selection in subsequent invasive BCI interventions (e.g., cortical electrodes or deep brain stimulation (DBS)).15 These features ensure their safety and effectiveness. The powerful spatial resolution and whole-brain field of view of fMRI provide an advantage in decoding refined cognitive states, providing an important pathway for developing diagnostic BCI tools or understanding the pathological mechanisms of neurological and psychiatric disorders.
3.2 Industry of invasive BCIs
Invasive BCIs have shown clinical value in areas such as motor function reconstruction, neurological disease regulation, and sensory restoration because of their high spatiotemporal resolution signal acquisition capability.41 Although several substantial technological barriers exist, invasive BCIs can have notable therapeutic effects. Figure 2 shows the timeline of the development of invasive BCIs.
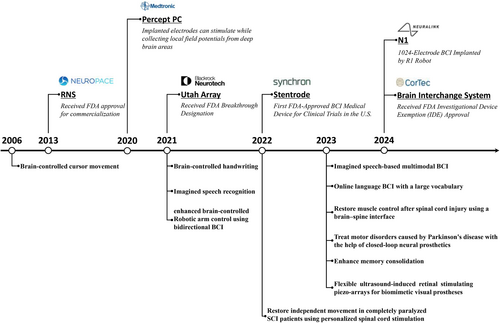
Timeline of the development of invasive brain-computer interfaces (BCIs).
3.2.1 Clinical application based on ECoG
Invasive BCI systems based on ECoG or intracortical microelectrodes have achieved notable breakthroughs in motion control for individuals with paralysis. In 2022, Synchron's endovascular electrode Stentrode was approved for an FDA Investigational Device Exemption for clinical trials. It is implanted near the motor cortex of patients with paralysis through a minimally invasive intervention, helping them decode neural signals and manipulate external devices to complete daily tasks such as text messaging and online shopping. Moreover, the BrainGate consortium has used cortical MEAs to achieve precise cursor control in patients with ALS and spinal cord injury. Blackrock's MoveAgain system uses a Utah array of electrodes to decode motion intentions and assist patients in operating assistive devices such as robotic arms.
Substantial progress has also been made in language decoding. An invasive BCI system developed by a joint team from Stanford University enabled a patient with paralysis to achieve a brain-controlled typing speed of 90 characters per minute with a corrected accuracy rate of >99% by decoding imagined handwriting. A team from the University of California, San Francisco successfully decoded text and synthesized speech directly from the brain activity of an individual with aphasia.42 In 2024, Neuralink announced the completion of its first N1 device human implantation, with 1024 channel electrodes implanted by surgical robots, aimed at restoring motor function in patients with quadriplegia.
3.2.2 Clinical application based on SEEG
Although SEEG technology is primarily used as the gold standard for preoperative evaluation of epilepsy, specifically to locate epileptic foci and map brain function, its implanted deep electrode array has also become an important BCI signal source.43, 44 SEEG electrodes can stably record LFP and high-frequency oscillation, providing a unique signal basis for BCIs. In recent years, BCI research based on SEEG has led to progress in decoding motor intentions (e.g., hand grasping or walking imagination) and language decoding, especially in exploring neural mechanisms for more natural, complex action intentions and advanced cognitive functions, such as semantic processing. The advantage of SEEG-BCI is that it can directly obtain signals from deep brain regions, such as the insula, cingulate gyrus, and medial temporal lobe, and the clinical implantation pathway is mature with controllable risks, providing an important platform for developing closed-loop controlled BCIs for deep brain diseases such as refractory epilepsy and certain psychiatric disorders.
3.2.3 Clinical application based on DBS
DBS technology is mainly being developed in the United States. A team from the University of Southern California achieved BCI enhancement of memory from the short-term (seconds) to the long-term (hours). The bidirectional BCI developed by the University of Pittsburgh improved the perception motion integration performance of prostheses. In addition, Rush University and the University of Southern California have developed electrical/ultrasound stimulation visual cortex prostheses. A study conducted at the University of California, Los Angeles confirmed that cortical stimulation can induce visual hallucinations in blind individuals without any complications.
DBS technology is increasingly being integrated with sensing capabilities to create closed-loop, intelligent systems. BCIs can enhance memory by demonstrating that a memory prosthesis system can facilitate human memory.45 The bidirectional BCI developed by the University of Pittsburgh improved the sensory-motor integration performance of prostheses.46 Currently, the DBS market is led by companies such as Medtronic and Abbott. NeuroPace's responsive neural stimulation (RNS) device can suppress epileptic seizures in real time. The Medtronic Perception PC system combines stimulation and LFP acquisition functions, promoting the development of closed-loop DBS. The AlphaDBS system from Newronika (Italy) optimizes Parkinson's disease treatment through adaptive algorithms.
3.3 BCI companies in the medical field
The BCI industry comprises a growing number of companies, ranging from startups to established medical device manufacturers, offering both non-invasive and invasive solutions. These companies target applications across motor control, rehabilitation, neurological disease monitoring, and emotional regulation, with firms from the United States, Europe, and China making significant contributions. Regarding invasive devices with regulatory approval, Synchron (USA), NeuroPace (USA), and Medtronic (USA) are key players in motor control or DBS. Prominent developers of invasive BCIs also include Neuralink (USA), Blackrock Neurotech (USA), and Paradromics (USA). The key non-invasive companies include Cognixion (USA), Kernel (USA), and g.tec (Austria), as well as Chinese firms such as BrainCo (QiangNao Technology) and Boruikang.
In the non-invasive domain, several companies focus on disease prediction and monitoring. Epiminder's Minder device (Australia) holds FDA breakthrough status for epilepsy monitoring. MJN Neuroserveis' CE-certified headphone device (Spain) uses AI for epilepsy prediction. Taiwan's Hondee Bio-Medical uses eight-channel EEG for diagnosing depression with over 80% accuracy. The sleep regulation devices include the former Dreem headband (USA), which uses auditory stimulation. Among Chinese-made devices, BrainCo's sleep devices and Zhejiang SoftWing's (Rouling) AirDream patches provide sleep intervention via EEG.
BCIs can also be used to treat neurodevelopmental disorders. EEG/fNIRS-based systems screen and improve ADHD/ASD through neurofeedback training. BrainMaster (USA) and Mind Media (The Netherlands) released early neurofeedback systems. Singapore's Neeuro developed digital training for ADHD. BrainCo launched the EEG-based Kaixingguo system for autism rehabilitation in China. Kernel offers portable fNIRS (Flow) and has demonstrated its use in evaluating the physiological effects of ketamine, for which the Flow system has received FDA approval. Table 2 lists examples of BCI companies focused on the medical field.
| Type | Application area | International | Domestic (China) |
|---|---|---|---|
| Non-invasive BCI | Motor rehabilitation and control | g.tec (Austria), Cognixion (USA), Meta (USA), MELTIN MMI (Japan) | Boruikang, Zhentai Intelligence, South China Brain Control, QiangNao Technology (BrainCo) |
| Brain disease monitoring and regulation | Epiminder (Australia), MJN (Spain), Neuroelectrics (Spain/USA) | Hongzhi Biomedicine | |
| Sleep and emotion monitoring and regulation | Dreem (USA), Neuphony (India), Interaxon (Canada), Trypylot (New Zealand) | Naolu Technology, Rouling Technology, QiangNao Technology, Hongzhili | |
| Neurofeedback training (commonly used for neurodevelopmental disorders) | BrainMaster (USA), Mind Media (The Netherlands), Neeuro (Singapore) | QiangNao Technology | |
| Cognitive monitoring and assessment | Kernel (USA) | Neurotalk | |
| Invasive BCI | Motor control | Synchron (USA), Neuralink (USA), BrainGate (USA), Blackrock (USA), Paradromics (USA) | Ningju Technology, Brain Tiger Technology, Jieti Medical |
| Motor rehabilitation | CorTec (Germany) | - | |
| Deep-brain stimulation (commonly used for tremors, epilepsy, and Parkinson's disease) | Medtronic (USA), NeuroPace (USA), Newronika (Italy), Bioinduction (UK) | Pins Medical, Jingyu Medical, Noway |
4 CHALLENGES OF BCIs IN THE MEDICAL INDUSTRY
4.1 Challenges
BCIs have potential in a wide range of medical applications. However, the main challenges for the practical implementation of BCIs include the lack of foundational technologies for non-invasive and invasive BCIs, the signal processing challenges associated with BCIs, the key components of BCIs, and the compatibility of BCI software and hardware.
BCI technology faces a dual development path: non-invasive BCIs are evolving through improved signal acquisition and AI-driven decoding technologies, whereas invasive BCIs are advancing toward minimally invasive solutions with enhanced biocompatibility and functionality, exemplified by innovations in flexible electrodes and vascular-interventional systems. These parallel developments suggest a potential convergence where hybrid approaches may combine the precision of invasive methods with the accessibility of non-invasive solutions. Overcoming core challenges, including signal stability, compatibility of BCI software and hardware, while ensuring cost-effective industrialization is critical for the widespread clinical adoption of these technologies.
The key advantage of BCI technology in the medical field is its ability to capture and analyze neural activity, providing revolutionary means for diagnosing and treating refractory neurological diseases. Invasive techniques, such as ECoG, SEEG, and MEAs directly contact neural tissue to capture neurophysiological signals with high spatiotemporal resolution, wide bandwidth, and strong anti-interference capabilities, laying a physical foundation for decoding complex neural information. This approach enables paralyzed patients to control the cursor on the screen, use mechanical prosthetics, and even achieve autonomous drinking using their minds. Invasive BCI combined with DBS or RNS has resulted in a personalized closed-loop treatment system for Parkinson's disease tremors and drug-resistant epilepsy. Non-invasive technologies, such as EEG and fNIRS, are used by a wider range of people and in various scenarios because of their non-invasiveness, ease of deployment, and safety. EEG, with its millisecond-level temporal resolution and portability, is widely used in neurorehabilitation training driven by motor imagery, objective assessment of mental states, and auxiliary treatment of pediatric neurodevelopmental disorders. Although limited by real-time performance, fMRI helps decode advanced cognitive functions and attain precise preoperative target localization for invasive interventions owing to its millimeter-level spatial resolution and whole-brain coverage capability.
However, several challenges exist in BCI technology. Signal quality is the main limitation of non-invasive BCIs. EEG is susceptible to severe interference from artifacts such as EMG and EOG. This technology is affected by skull attenuation effects, resulting in low spatial resolution and difficulty in locating deep-brain activity. fNIRS and fMRI exhibit inherent delays (in seconds) in the hemodynamic response, making it difficult to meet the requirements of real-time and rapid control. In contrast, the large size, high cost, and harsh usage environment (magnetic shielding rooms) of fMRI and MEG devices limit their practical application. Invasive BCIs face safety and long-term stability issues. Surgical implantation is accompanied by risks of infection, bleeding, and tissue damage. Moreover, the biocompatibility of long-term implants needs to be addressed, as signal attenuation or even failure may result from scar tissue wrapping around electrodes and glial cell proliferation. Packaging reliability, high power consumption, and bandwidth limitations for high-channel wireless data transmission also constrain the widespread application of invasive BCIs.
4.2 Development requirements
Given the practical needs of clinical applications, significant advancements are required in several key directions to accelerate the clinical implementation of BCIs. First, efforts should be made to develop next-generation implant materials with enhanced biocompatibility. Some examples include flexible conductive polymers, biomimetic coatings, and minimally invasive or interventional deployment schemes, such as intravascular electrodes. The focus should be on optimizing the long-term stability of the electrode-tissue interface by reducing gliosis, ensuring stable contact between intravascular electrodes and tube walls, and reducing surgical risks and long-term complications. Second, a fusion of multimodal sensing technology is needed, for example, by combining EEG and fNIRS. Advanced signal processing algorithms are required to increase the anti-interference ability, decoding accuracy, and practicality of non-invasive systems. Third, an efficient, low-latency, and highly reliable system is needed to seamlessly integrate high-fidelity neural signal recording, real-time intent decoding, and precise regulation output. In the future, if novel methods for acquiring or decoding neural signals are developed that enable non-invasive BCIs to achieve signal quality comparable to that of invasive techniques, it will propel BCI technology to leapfrog in development. In addition, a sound ethical foundation and a robust regulatory framework for long-term safety and effectiveness are also necessary to ensure the success of BCI technology.
5 CONCLUSION
This study has examined the pivotal challenges hindering the medical industrialization of BCIs. These challenges include the lack of foundational technologies for non-invasive and invasive BCIs, the signal processing challenges associated with BCIs, the key components of BCIs, and the compatibility of BCI software and hardware. Addressing these complex challenges requires a concerted, multidisciplinary effort. Success hinges on deep collaboration across neuroscience, engineering, materials science, and clinical medicine. The development of core enabling technologies (e.g., high-performance biocompatible interfaces, robust wireless systems, intelligent decoding algorithms) should be prioritized. Furthermore, large-scale longitudinal clinical trials should be conducted to demonstrate efficacy and cost-effectiveness, and establishing positive ethical support should be established. These approaches can enhance the competitiveness of BCI productions and facilitate the integration of this technology into the medical industry. In conclusion, BCIs have the potential to improve medical technology and the quality of human life; however, further technological breakthroughs are still needed.
AUTHOR CONTRIBUTIONS
Qi Chen: Writing—original draft and editing. Sha Zhao: Writing—original draft and editing. Wei Wei: Writing—review and editing. Tianyu Zhao: Writing—review and editing. Rui He: Writing—review and editing. Sishu Zhou: Writing—original draft and editing. Zhenhang Yu: Writing—review and editing. All authors have read and approved the final article.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (62476240).
CONFLICT OF INTEREST STATEMENT
Although Sishu Zhou is currently employed by Zhejiang ZJU-XITOU Brain Machine Intelligent Technology Co. Ltd, there are no conflicts of interest regarding his involvement in this paper and his work at the company. The other authors declare that they have no conflicts of interest.
ETHICS STATEMENT
Ethics approval was not needed in this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




