A comprehensive review of hydrogel strategies for repairing peripheral nerve injuries
Shicheng Jia, Hongfa Zhou and Jiayou Chen contributed equally to this work and shared the first authorship.
Abstract
As an etiological factor underlying physical and mental disability in humans, peripheral nerve injuries (PNIs) can induce pain, sensory impairment, and disability. Despite their regenerative ability, peripheral nerves cannot self-repair after severe defects. While nerve grafting is the gold standard for the treatment of PNIs, it is limited by graft versus host reactions, surgical complications, and limited donor nerves. As the field of material science continues to develop, hydrogels have been proposed for use in PNI repair after their biomodification, targeted modification, or loading with biological factors and cells. This article reviewed research advances in hydrogels used for PNI repair, including simple hydrogels and composite functionalized hydrogels loaded with biological factors and cells. Based on the findings from these reviews, we determined that further clarification of the mechanisms of action for hydrogels and loaded biological factors in modulating cellular functions is necessary. In addition, there is a need to further explore the synergistic effect of novel functionalized hydrogels with other biological, physical, or biochemical factors. While clinical trials are still limited, scientific efforts are expected to promote the application of hydrogels in the field of PNI repair.
Key points
What is already known about this topic?
-
Peripheral nerve injury is one of the etiological factors underlying physical and psychological disorders in the body. With the development of new biomaterials, the nerve regeneration based on the interdisciplinary disciplines of tissue engineering, regenerative medicine, and materials science has received widespread attention Numerous experimental studies have explored the important role of different hydrogel types in therapeutic peripheral nerve repair.
What does this study add?
-
The study adds an up-to-date, systematic, and comprehensive review of the field, including the history of hydrogel development for peripheral nerve repair, a systematic summary of the different types of base materials, the hydrogels derived from these materials, and the various therapeutic components that can be used for delivery by hydrogels.
1 INTRODUCTION
Peripheral nerve injury (PNI) can cause pain, sensory disorders, and disability and is one of the etiological factors underlying physical and psychological disorders in the body.1-3 Currently, the number of patients with PNIs due to trauma, tumor removal, and diabetes is increasing.4 The results of one epidemiological study revealed that among 70,000 hospital inpatients, nearly 20% of them suffered from peripheral neuropathy.4 Although nerve grafting is the gold standard for the treatment of PNIs, it is limited by graft versus host reactions, surgical complications, and few donor nerves.5, 6 However, with the development of new biomaterials, the repair of PNIs and nerve regeneration based on the interdisciplinary disciplines of tissue engineering, regenerative medicine, and materials science has received widespread attention.7, 8
Normal myelinated nerves are surrounded by Schwann cell (SC) membranes, forming an onion-skin-like myelin sheath. This structure ensures the transmission of electrical signals and maintains normal neurophysiological function. However, the death of neurons and glial cells, vascular ruptures, and secondary injuries may occur after a PNI. These pathological processes can be accompanied by myelin destruction, distal axonal swelling, and destruction in a process called Wallerian degeneration. In these circumstances, various inflammatory cells are activated, accumulating at the injury site and engulfing most of the myelin debris and fat. After a PNI, neurons receive a retrograde electrical activity signal in the form of high-frequency action potential bursts, which open calcium channels, allow an influx of Ca2+, and initiate the JUN-kinase cascade. This process affects transcription9 and changes the cell from a myelin phenotype to a regenerative phenotype. These cells also exhibit the up-regulation of molecules that contribute to the regeneration process, such as the cell adhesion molecule (CAM), glial fibrillary acidic protein, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor, basic fibroblast growth factor, and neurotrophin-3.10-12 After a PNI, factors such as retrograde transport signaling disorders, Ca2+ influx, and exposure of the injured nerve end to degenerative and inflammatory environments stimulate proximal nerve regeneration.13 However, despite the regenerative ability of peripheral nerves, they cannot achieve self-repair in cases where a defect is over 10 mm.14 If the PNI is severe, numerous proliferating inflammatory cells, Schwann cells, and collagen cells secrete extracellular matrixes (ECMs) to form dense scars, which can eventually lead to the failure of peripheral nerve regeneration.3, 15, 16 PNIs involve multiple molecular mechanisms, including cytokines, growth factors, CAMs, neurotransmitters, and other molecular signaling molecules (summarized in Table 1 17-30). Therefore, based on the pathophysiological process, an ideal peripheral nerve repair material should be developed with the following properties: (1) favorable biocompatibility to avoid graft immune rejection and the toxic effects of degradation products on the organism31, 32; (2) conductivity and the ability to promote neural regeneration, which can guide the growth and lengthening of nerve fibers and ensure the maintenance of damaged nerve function while satisfying the regeneration of damaged peripheral nerves proximal to SCs to repair myelin33; (3) a suitable pore size (ideally 10–20 μm) to allow nutrient entry and metabolic waste discharge while preventing fiber scar formation34; (4) suitable mechanical strength, ductility, and flexibility to prevent nerve tissue from being compressed35-37; (5) a suitable microstructure for cell adhesion and interaction34, 37; (6) and stable components to prevent reactions with various biological factors that could destroy the structure of the material or induce the loss of activity of biological factors.34, 38
| Types of molecules | Molecules | Mechanism of action | Activation pathway | Comments | Reference |
|---|---|---|---|---|---|
| Growth factors | Nerve growth factor | The main function is promoting the survival and maintenance of neurons. It can also promote the formation of synapses in neurons, stimulate the growth of neuronal axons, and inhibit inflammation | Ras/PI-3K/AKT pathway | Trk tyrosine kinase receptor and the p57 neurotrophin receptor substantially participate in regulation. Functional communication between the signaling pathways appears to be a key process that determines how the nervous system develops and repairs itself after an injury | [17-20] |
| MEK/MAPK pathway | |||||
| JNK-p53-Bax pathway | |||||
| NF-κB | |||||
| Brain-derived neurotrophic factor | Similar to NGF | Similar with NGF | BDNF exerts integrative effects on neuronal survival, axon growth, and synaptic plasticity by regulating multiple TrkB-mediated signaling pathways | [21] | |
| Insulin-like growth factor | IGF-1 prevents SC cell apoptosis, promotes axon growth, inhibits neuroinflammation, and reduces the rate of denervation-induced muscle atrophy | PI3K/Akt pathway | There is an urgent need for an optimized delivery system that can sustainably deliver bioactive IGF-1 to target tissues in a safe and clinically practical manner. The optimal dose range of IGF-1 varies widely depending on the delivery mechanism | [22] | |
| ERK pathway | |||||
| Akt/mTOR pathway | |||||
| TGF-ß | Regulates SC viability | Smad-2 pathway | TGF-β has a “bipolar function” (i.e., stimulation and inhibition) in PNI repair, including the regulation of cell survival, growth, proliferation, differentiation, migration, neuroinflammation, and neurotrophic factor secretion | [23-27] | |
| cAMP pathway | |||||
| c-Jun pathway | |||||
| External stimulation pathway | |||||
| NF-κB | |||||
| Regulates inflammation and immunity following nerve injury | ALK4-ERK | ||||
| M2a selective activation | |||||
| Drives perineurial glial bridging | Smad-3 | ||||
| Hepatocyte growth factor | Promotes the migration of SCs | c-Jun pathway | More research is needed to identify its mechanism and functions | [28] | |
| ERK/AKT pathway | |||||
| HGF/c-met pathway | |||||
| Sox10 pathway | |||||
| Fibroblast growth factor | Classic mitogenic and neuroprotective activities | MAPK/ERK pathway | FGFs capable of binding to their respective receptors and exerting their biological effects by activating different types of downstream signaling pathways. Despite their ability to increase the survival of SCs and promote axonal regeneration and myelin sheath regeneration after a PNI, they have numerous drawbacks that must be overcome to optimize their properties and achieve optimal treatment for axonal regeneration and functional recovery | [29] | |
| Promotes angiogenesis | JNK/c-Jun pathway | ||||
| Stimulates cell proliferation, migration, and differentiation | PI3K/Akt/mTOR or PI3K/Akt/GSK3β pathway | ||||
| Cell adhesion molecules | Integrin | Developes tissues, nervous system formation, immune responses, and the regeneration of axons | ATF3/CREB pathway | Signaling pathways such as integrin/FAK are more common and believed to initiate the regenerative process. Multiple downstream integrin pathways are recognized as potential therapeutic targets for restoring damaged axonal function. However, there is no effective and affordable therapeutic approach for the restoration of axonal function | [30] |
| FAK pathway | |||||
| GSK3β–CLASP/APC pathway |
Hydrogels are novel materials that repair PNIs after biomodification, targeted modification, or loading with biological factors or cells. The neuroregenerative activity of hydrogels can be enhanced through biomodification, which is achieved by physically and chemically linking bioactive groups and active peptides or loading bioactive factors. Targeted modification involves conjugating specific targeting molecules to augment the hydrogel components' regulation of particular molecular pathways or specific cells. These approaches contribute to the heightened neurorepair potential of hydrogels.
Hydrogels are characterized by three-dimensional (3D) networked solid structures of hydrophilic polymers capable of absorbing biologically produced fluids.39, 40 They can respond well to changes in physicochemical factors in the environment due to their controlled cross-linked density and chemical properties41 and can deliver biological factors or cells through complex kinetics. In addition, some of these gels can be designed with various morphological patterns due to their injectability, such as hyaluronic acid (HA), hydroxyapatite, and polylactic acid, which allow them to be used in nonstandard geometrical structures for scaffolding treatment, especially in tissue regeneration.42, 43 Moreover, hydrogels are particularly suitable for soft tissue repair in vivo, such as in the brain and spinal cord,44, 45 and their excellent properties have promoted their use in PNI repair. In this review, we summarized the properties of hydrogels with different components and their advantages and shortcomings when used in PNI repair. Research advances in hydrogel-based PNI repair biomaterials were systematically reviewed, including simple hydrogels, composite hydrogels loaded with biological factors, and modified hydrogels loaded with cells. This review is expected to provide comprehensive and systematic insight into the application of hydrogels in biomedical engineering.
In the late 19th to early 20th century, hydrogels were extensively researched as growth-permissive substrates within conduits that would enhance regeneration across long-segment nerve defects.46 Compared to an empty tube or one containing physiological saline solution, nerve guidance conduit (NGC)-filled fibrin matrices, laminin-containing gels, collagen, and HA have been shown to perform faster axonal regeneration.47-50 Due to hydrogels' superior drug delivery performance and biocompatibility, biomaterials based on hydrogels loaded with bioactive substances and cells have been progressively developed. Incorporating growth factors, cell adhesion peptides, and glial cells such as SCs have augmented hydrogels' promotive effect on neural regeneration.51-54 Given the challenges in obtaining autologous SCs in a clinical setting, recent research on cell-loaded hydrogels for PNI regeneration has primarily focused on mesenchymal stem cells (MSCs), which are more accessible. This shift aims to enhance the translatability of research to clinical applications.55, 56 Responsive hydrogels can adjust their characteristics when reacting to external stimuli, such as temperature, pH, and optics, thereby regulating cell signaling and behaviors that align with the requirements of the neural repair process.57-59 Developing these smart-responsive hydrogels hold potential for advancements in PNI regeneration.60 Based on the continued progress in the scientific community's understanding of PNI repair, we have summarized research milestones in Table 2. This work further aims to analyze recent trends in the design of hydrogels for peripheral nerve regeneration, focusing on the role of hydrogel ingredients to derive guidelines for optimal fabrication.
| Publication year | Implications of landmark research and research/articles | Title of reference |
|---|---|---|
| 2006 | Guidance channels with varying permeability (that involve electrically active channels) and degradable guidance channels improve regeneration compared to no intervention but rarely approach or match the performance of autografts when gaps are 10 mm or longer. There is a consensus that bridging long peripheral nerve gaps involves filling NGCs with scaffolds/constructs that promote regeneration | Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy |
| 2009/2006 | NGCs filled with fibrin, laminin, collagen, or polymer fibers perform more rapid axonal regeneration than a hollow conduit or a conduit containing physiological saline solution | Thin-film enhanced nerve guidance channels for peripheral nerve repair. Luminal fillers in nerve conduits for peripheral nerve repair |
| 2013 | In NGCs, engineered Schwann cells support robust neuronal regeneration across gaps | Engineered neural tissue for peripheral nerve repair |
| 2003 | Bioactive molecules enhance axonal regrowth and functional recovery after a peripheral nerve injury | Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube |
| 2015 | Given comparable growth promotion and less high-risk access to Schwann cells, NGCs filled with stem cells have more potential for clinical translation than cells only | Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve |
| 2020 | Responsive hydrogels can adjust their characteristics in response to external stimuli, such as temperature, pH, and electrical and photomechanical stimulation. This provides the hydrogel with injectability and maneuverability, and regulates cell signaling and behaviors that align with the neural repair process | Conductive Hydrogel for a photothermal- responsive stretchable artificial nerve and coalescing with a damaged peripheral nerve |
2 OVERVIEW OF HYDROGELS WITH DIFFERENT COMPONENTS FOR PNI REPAIR
Common hydrogels are divided into two categories: natural- and artificial-component hydrogels. Natural components have better biocompatibility but poorer mechanical properties and less rigidity than artificial components. Common natural components comprise alginate, collagen, gelatin, and HA. In contrast, artificial components consist of polyethylene glycol (PEG) derivatives, polycaprolactone (PCL), and polyvinyl alcohol (PVA). While these components have been developed to supplement natural hydrogels, their biocompatibility needs to be further verified.61 Hence, natural and artificial materials have both advantages and disadvantages in PNI repair (Table 3 62, 64, 65, 69, 72-80).
| Summary of materials | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Materials | Chemical structural formula | Source | Water solubility | Mechanical strength | Viscosity | Biological activity | Advantages | Disadvanteages | Reference | |
| Natural hydrogel | Polypeptide |  |
Chemically synthesized or extracted from natural proteins | Depends on the composition of amino acids | Depends on the composition, sequence, and structure of amino acids | Depends on molecular weight as well as solvent condition and structure | Better | Structural versatility; Easily to be synthesized | Structural complexity; | [62] |
| Molecular mass; | ||||||||||
| Inhomogeneity; | ||||||||||
| Difficult to disclose the interaction of the nanocarriers with biological interfaces | ||||||||||
| Alginate |  |
Extracted from seaweed | Better | Worse | Better | Better | Excellent biocompatibility; | Poor mechanical properties; | [63] | |
| Biodegradability; | ||||||||||
| Low toxicity; | Lack of cellular interaction | |||||||||
| Renewable prospects | ||||||||||
| Collagen |  |
Extracted from animal skin, bone and connective tissue | Better | Better | Worse | Better | Excellent biocompatibility; | Poor mechanical properties; | [64] | |
| Thermal reversibility | Potentially immunogenic; | |||||||||
| Expensive | ||||||||||
| Gelatin | Uncertain structure | Extracted from animal skin, bone and connective tissue | Worse | Better | Worse | Better | Excellent biocompatibility; | Poor mechanical properties | [65] | |
| Ease of gelation | ||||||||||
| Agarose |  |
A natural polysaccharide derived from seaweed | Better | Worse | Better | Better | Excellent biocompatibility; | Poor mechanical properties; | [66] | |
| Low toxicity; | ||||||||||
| Low cost; | Poor thermal stability | |||||||||
| Ease of controllable gelation | ||||||||||
| Cellulose | 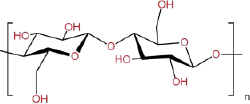 |
Extracted from plant cell walls | Worse | Better | Worse | Better | Excellent biocompatibility; | Poor mechanical properties | [67] | |
| High viscosity and viscoelasticity | ||||||||||
| Hyaluronic acid | 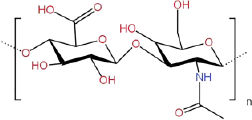 |
Extracted from animal tissues or fermented by microorganisms | Better | Worse | Better | Better | Excellent biocompatibility; | Difficult to combine with hydrophobic drugs | [68] | |
| Low toxicity; | ||||||||||
| Moisturizing properties | ||||||||||
| Artificial hydrogel | Polyvinyl alcohol |  |
Polymerization of vinyl compounds | Better | Controllable | Controllable | Bioinert material | Controllable physicochemical properties; | Lack of cellular interaction; | [69] |
| Good mechanical properties | Poor biocompatibility | |||||||||
| Polyethylene glycol | 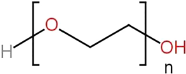 |
Polymerization of ethylene glycol monomer | Better | Controllable | Controllable | Bioinert material | Effective fusogen- mediating cell fusion; | Good mechanical properties; | [70] | |
| Good mechanical properties | Potentially immunogenic; | |||||||||
| Toxicity; | ||||||||||
| Polycaprolactone | 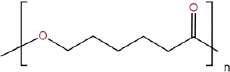 |
Polymerization of caprolactone monomer | Worse | Controllable | Controllable | Better | Excellent biocompatibility; | Lack of cellular interactions; | [71] | |
| Controllable physicochemical properties; | ||||||||||
| Good mechanical properties | The degraded products may cause local inflammation | |||||||||
| Summary of hydrogels | |||||
|---|---|---|---|---|---|
| Main components | Name of hydrogels | Modification | Experimental model | Mechanism of action | References |
| Alginate | Poly-3-hydroxybutyrate (PHB) conduits with hydrogel | Poly-3-hydroxybutyrate | SCs and rat model | Enhances the regeneration rate and presents an additive effect when SCs and fibronectin are combined | [194] |
| Bilayer P-CA conduit with hydrogel | N, N′-disuccinimidyl carbonate | Rat model | Promotes the adhesion and proliferation of nerve cells, provides the maximum tensile strength of the conduit during surgery, and promotes the migration of SCs along the axon | [72] | |
| SA/CMCS/PPy hydrogel | D-glucono-δ-lactone (GDL) and superfine calcium carbonate | PC12 cells | Promotes the adhesion and growth of neural cells with electrical conductivity and biological activity | [74] | |
| Chitosan | Methacrylate-chitosan hydrogel | Methacrylate | miPSCs | Supports the maintenance of iPSCs and their differentiation into neuronal phenotypes | [195] |
| Hydrogel-based chitosan tubes | - | Dorsal root ganglion (DRG) cells | Supports nerve cell adhesion and neurite outgrowth | [76] | |
| Peptide | Multidomain peptide nano-hydrogel | Multidomain peptide | Rat model | Enhance macrophage recruitment to the injury site, neurite outgrowth, and multicellular pro-regenerative responses in PNIs | [79] |
| Peptide amphiphile (PA)-based hydrogels | Peptide amphiphile | SCs and mouse model | Promotes the spread, proliferation, and migration of cells. Supports angiogenesis without causing inflammation or a foreign body immune response | [73] | |
| RADA 16-MIX self-assembled hydrogel | IKVAV and RGD | Rat model | Induces the regeneration of axons and the immigration of SCs | [196] | |
| Self-assembling peptide (SAP) nanofiber hydrogel | Self-assembling peptides (SAP) with IKVAV and KLT sequences | SCs and rat model | Accelerates nerve healing and enhances morphological repair | [197] | |
| Gelatin | GelMA-pHEMA hydrogel | Methacrylated gelatin-poly(2-hydroxyethylmethacrylate) | SCs | Increase the mechanical strength and promote the adhesion of SCs | [75] |
| PLCL conduit with swellable gelatin hydrogel | Electrospun poly (lactide-co-ε-caprolactone) | Rat model | Provides conducting guidance and serves as a reservoir that can incorporate and release bioactive molecules to promote nerve regeneration | [77] | |
| Tiple-layered high-resolution EHD printed PCL-gelatin hydrogel | Electrohydrodynamic jet | PC12 cells | Provides potential to fabricate mechanically tunable triple-layered conduits with favorable neuronal precursors and vascular cell compatibility | [78] | |
| Decellularized ECM | Electrospun nanofiber modified PDGNM hydrogel | Electrospun nanofibers | SCs and rat model | Facilitates directed axonal extensions and SC migration in vitro | [198] |
| DNMS/GDNF@DNMG | Glial-derived neurotrophic factor | Rat and beagle model | Enhances nerve regeneration and functional recovery | [193] | |
2.1 Natural-based component hydrogels in PNI repair
2.1.1 Peptides
Peptides are the intermediates of protein hydrolysis. They consist of amino acids and can be enzymatically degraded in vivo, with the resultant products, also being amino acids, readily assimilated by cells. Consequently, they exhibit high biocompatibility within biological systems.81 Under specific conditions, certain peptides can self-assemble to form ordered high-dimensional structures, providing scaffolding for cell adhesion and growth.82 This property offers potential in designing and fabricating hydrogels with designated functionalities. Additionally, peptides can mimic the functional domains of natural proteins, providing peptide-based hydrogels with specific bioactive properties.83 Hence, they have a high potential for tissue engineering, biological factors delivery, and biosensing applications.
Peptide hydrogels have been extensively investigated. However, the synthesis of self-assembled functional peptide hydrogels with corresponding protein sequences based on the molecular structure of known high-performance materials remains unclear. Wei et al.84 developed a silk fibroin peptide (SF16) hydrogel containing a filamentous protein sequence, which could support PC12 cells in secreting matrixes, promoting cell proliferation in damaged neural sites, regulating neural-associated cell differentiation, and enhancing neurite growth. In a self-assembled 3D peptide hydrogel with dual functionality achieved by the adhesion protein modification motif IKVAV, the brain-derived NGF peptide site RGI was able to significantly enhance the adhesion of SCs to the material, promote the expression of NGFs, BDNFs, ciliary neurotrophic factor, peripheral myelin protein 22, neuropilin 2, and other related genes, and successfully repair a 10 mm sciatic nerve defect in vivo.52 Owing to their derivation from proteins and the multiple binding sites of peptide hydrogels, a synergistic effect between IKVAV and RGI was achieved, promoting axonal and myelin regeneration and accelerating motor function recovery.52 Peptide co-assembly with mimic peptide epitopes has also been proposed to functionalize hydrogels. Lu et al.51 used this approach to obtain hydrogels modified with BDNF and vascular endothelial growth factors (VEGFs), which provided a 3D neurovascular microenvironment for the growth of endothelial and neuronal cells and similarly promoted PNI repair. Compared with self-assembled and co-assembled peptides, self-sorting peptides can selectively interact with specific ligands to produce complex assemblies with precise components.85 While it has been confirmed that these hydrogels can promote tissue self-healing, their application in PNI repair requires further verification.86
Peptides can assemble into high-dimensional structures that support cell adhesion and proliferation.87 Upon degradation, they yield amino acids that nourish cells. By mimicking active sites, peptides modulate cellular behavior, thus demonstrating strong interactions with cells and emerging as promising materials for tissue engineering.88 However, while peptides are more stable than proteins, they still exhibit sensitivity to temperature, pH, and mechanical stimuli.89 As biomaterials for repair, their limited stability can result in the leakage or premature release of encapsulated substances during the repair process. Furthermore, the metabolic instability of their degradation products may induce inflammation.90-92 Therefore, it is necessary to further observe the release behavior of peptide hydrogels in real-time with a monitoring device and examine their efficacy on the encapsulation of substances.
2.1.2 Alginate
Alginates are biologically inert materials composed of homopolymers, and the G residues within them can be cross-linked to produce hydrogels.93 They can then be chemically decomposed and reconstituted into a nanoporous structure like a basement membrane, which physically restricts the encapsulated cells and affects their migration, proliferation, and differentiation.93, 94 However, after alginates absorb a large volume of water and form gels, they are prone to deformations by movements or external pressures due to their high hydrophilicity. These deformations can damage the neural stem cells they carry or the ones at the implantation site.61 Jin et al.95 prepared a new biosafe hydrogel with graphene and sodium alginates that exhibited favorable conductivity and mechanical properties, and anti-inflammatory functions. It promoted the release of neurotrophic substances and up-regulated the expression of growth factors while simulating the neural growth microenvironment. Another study used alginates and collagen to prepare hydrogels containing derived exosomes critical in regulating neural differentiation. The alginates were shown to have good biocompatibility that facilitated the growth of SCs within its microstructure and provided conditions for the secretion of neurotrophic factors acting on tropomyosin receptor kinase A receptors.96 Raimondo et al.97 revealed that injectable alginate hydrogels could load VEGFs and insulin-like growth factor-1 and transport them to damaged sites in vivo to promote the regeneration of muscle fibers and peripheral nerves. Alginate also exhibits pronounced hydrophilicity, enabling it to form hydrogels that structurally resemble the ECM.93 This characteristic, and its excellent biocompatibility, supports cell adhesion, proliferation, differentiation, and controlled drug release.98 In addition, the native polysaccharides of alginate possess inherent anti-inflammatory properties. However, its limited mechanical strength restricts the standalone application of pure alginate hydrogels.93 Still, through different profile designs and material modifications, alginate hydrogels could obtain favorable physicochemical properties, bioactivity, and biological factor loading performance, which would benefit their use in PNI repair.
2.1.3 Collagen
Collagen is the most abundant component in ECMs. It can be converted to a hydrogel via self-fibrosis under physiological conditions achieved through changing external conditions, such as pH and temperature.99 Thus, collagen hydrogels are bionanomimetic materials that can mimic the physiological cellular microenvironment and are widely used in regenerative medication and PNI repair. Masand et al.100 proposed using polysalivary acid and the human natural killer cell epitope HNK-1 to prepare functionalized collagen hydrogels. They discovered that these hydrogels promoted PNI repair by improving axon number, motor neuron targeting, and axon myelination. Zhang et al.101 improved the physical structure of collagen hydrogels via the 3D method to fabricate loaded gingiva-derived mesenchymal stem cells (GMSCs). Their results showed that stem cells could migrate and integrate into the ECM of natural neural stem cells through the hydrogel. This significantly promoted the recovery of neural function and axon regeneration under the regulation of the Notch signaling pathway. As a natural component of the ECM, collagen hydrogels exhibit superior biocompatibility compared to alginate ones.102 Nevertheless, like alginates, collagen hydrogels are highly sensitive to mechanical stimuli, mainly exhibiting limited tensile resistance. Furthermore, being a natural protein, collagen is readily recognized and degraded by matrix metalloproteinases (MMPs) with collagenase activity, leading to rapid in vivo degradation.103 This rapid breakdown poses challenges for prolonged processes such as neural repair. Therefore, various chemical cross-linking strategies might enhance their properties, rendering them more suitable for PNI repair.
2.1.4 Gelatin
Gelatin is a decomposed product of collagen that does not have a triple helix structure with the same molecular components as collagen and is considered an ideal collagen substitute.104 Some researchers have developed a 3D photo-cross-linked, gelatin-based hydrogel that can encapsulate SCs. This hydrogel exhibits high viscosity and strength, and SC viability can be retained. Moreover, it promotes SC proliferation, neurite extension, and glial cell recruitment after being filled into damaged areas.105 Compared with conventional nerve conduits, the double network of gelatin, when combined with alginate-loaded netrin-1 hydrogels (as prepared by Huang et al.106), not only targets axon pathfinding and neuronal migration but effectively induces cannula formation, which makes it a potentially superior material for neural repair than autologous grafts. In addition, gelatin can be biodegraded by cell-derived enzymes (e.g., MMPs), which contribute to cell-mediated matrix remodeling.104, 107 As gelatin is derived from collagen, it offers biocompatibility and mechanical properties comparable to collagen, with the added benefits of reduced production costs and adjustable degradation rates.103, 108 However, gelatin hydrogels have the same disadvantages as collagen in terms of mechanical strength. Compared to collagen hydrogels, gelatin ones form pores larger than cells. While these scaffolds provide a 3D structure at the macroscopic level, the large pore size means individual cells effectively experience a 2D culture microstructure.94 Still, gelatin has more prospective applications than collagen for preparing biological factor- and cell-laden materials.
2.1.5 Hyaluronic acid
HA is extremely biosafe and has strong anti-inflammatory abilities and influence on cell migration and differentiation due to its binding sites with cells overexpressing CD44.61 This feature is the basis for preparing relevant hydrogel materials established on neural regeneration promotion theories, such as recruiting SCs. Compared with other natural macromolecules, HA has poor autonomic cross-linking ability,61 which needs stricter and more requirements for surface modification, performance improvement, and development applications. Huang et al.109 demonstrated that a simple HA-laminin hydrogel, acting as a hollow nerve conduit, can increase the thickness of regenerating fiber sheath membranes by inhibiting functional axonal loss and increasing the density of regenerating nerve fibers. Liu et al.110 found that photo-cross-linked HA methacrylate hydrogels carrying stem cell-derived exosomes could promote PNI repair and downregulate the expression of inflammatory factors, such as interleukin-1 β and tumor necrosis factor. HA hydrogels with an elastic modulus of 0.78 kPa can promote exosome release through their weak rigidity, which could provide a new direction for developing HA-based hydrogels. In addition, HA hydrogels with a high molecular weight (more than 107 Da) have stronger anti-inflammatory and anti-angiogenic effects than low ones.61 Overall, HA hydrogels exhibit superior bioactivity compared to collagen, gelatin, and sodium alginate. While they suffer from the mechanical limitations inherent to natural hydrogels, their mechanical properties can be enhanced by altering cross-linking methods, incorporating nanomaterials, or blending with high-performance polymers.94, 111 It might also be feasible to improve the function of these materials by modulating their intrinsic properties, such as molecular weight. Moreover, proper cross-linked groups could be selected to modify these materials when HA is used to promote PNI repair.
2.1.6 Agarose
Agarose is a natural, biocompatible polysaccharide extracted from red algae. It can self-coagulate at 37°C without cross-linking agents, so its toxicity can be avoided. This polymer also has a high water absorption capacity, strong biological activity, and characteristics similar to natural ECMs, which can promote cell growth, differentiation, and proliferation.112 Agarose-made scaffolds are widely used in nerve defects but are not enriched in ECM molecules, cells, or factors to enhance growth.113 Therefore, growth factors, stem cells, and agarose can form a composite scaffold to enhance the repair effect. Gao et al. implanted a BMSC-BDNF-multichannel agarose-guided scaffold for PNI regeneration into a 15-mm-long rat sciatic nerve space, a composite scaffold that mimicked the 3D structure of the peripheral nerve. The BMSCs secreted matrix, fibronectin, collagen, and BDNF, which promoted axonal growth and successfully guided long-distance linear axonal regeneration.114
2.1.7 Cellulose
Cellulose is the most abundant biopolymer in plant cell walls and is produced by animals, fungi, and bacteria. It has good biocompatibility and biological activity, and its nonbiodegradable characteristics make it suitable for nerve injury recovery.115 Zheng et al. used oxidized hydroxyethyl cellulose and chitosan (CS) to cross-link polymerization. Within a specific range, the mechanical properties and porosity of polymeric materials are directly proportional to the proportion of oxidized hydroxyethyl cellulose, and the swelling behavior and degradation rate of polymeric materials decreases with an increase in oxidized hydroxyethyl cellulose.116 Therefore, cross-linking cellulose with other hydrogels is helpful to improve the characterization of the materials.
Studies have also shown that electrical stimulation can promote nerve regeneration.117 Asiaticoside liposomes can interfere with the DNA synthesis of fibroblasts and inhibit their differentiation and proliferation, reducing collagen synthesis and inhibiting the development of scar tissue.118, 119 Overall, the best cell proliferation, axon growth, and cell differentiation occur in this hydrogel group.
2.1.8 Decellularized extracellular matrix (dECM)
dECM has been widely used as a reliable alternative material for PNI repair in clinical surgery. Since the decellularization technique can preserve numerous bioactive components in the ECM, such as polysaccharides, proteins, and growth factors, dECM-based biomaterials can preserve the intrinsic microenvironment of tissues, promote the proliferation and differentiation of SCs and induce neural regeneration.120 However, its application has some limitations, including poor preservation effects of active components in the initial preparation, sterilization, and preservation processes, poor reproducibility of homogeneous dECM, and a weak ability to continuously release NGFs. Given these restraints, the material was first used as a lumen filler for nerve conduits or a transplantation vehicle for SCs.121, 122 More recently, however, dECM has gradually been used in hydrogels. Commonly used dECM hydrogels are of porcine neurogenic origin and exhibit favorable in vitro properties in promoting the axonal growth of dorsal root ganglion (DRG) cells and the myelination of axons by SCs123 (Figure 1). Meder et al.125 developed a decellularized, porcine nerve-derived hydrogel filler. They evaluated the recovery of peripheral innervation functioning 24 weeks post-injury and found improved distribution of peripheral nerve matrixes within the catheter, enhanced electrophysiological responses, and more nascent axons than in the control group. Xue et al.126 proposed a theory related to the facilitative effect of the synergistic action of dECM hydrogels and their components on the behavioral regulation of DRGs and SCs. However, despite their high ductility, dECM hydrogels lose the natural structure of intact ECMs and are susceptible to bacterial contamination.
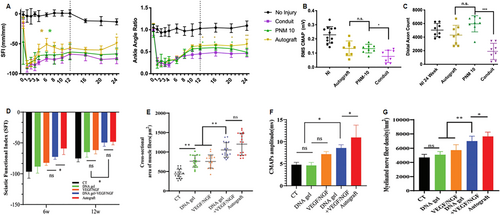
The potential of DNA and dECM hydrogels to accelerate the regeneration of peripheral nerves. (A) A comparison of SFI and ankle angle treatment types over 24 weeks. Gold asterisks denote a significant difference between autografts and PNM-10, and green asterisks denote a significant difference between PNM-10 and the conduits. (B) CMAP at 24 weeks comparing treatment types. (C) Axon counts for each group. (D) SFI values of each group at 6 and 12 weeks after surgery. (E) Quantitative analysis of the mean cross-sectional area of muscle fibers from the injured side. (F) Quantitative analysis of CMAP amplitude in each group at 12 weeks after surgery. (G) Quantitative analysis of the density of myelinated nerve fibers evaluated by toluidine blue staining. Panels reproduced with permission from (A) to (C)124, Royal Society of Chemistry; (D)–(G)125, Authors. CMAP, compound muscle action potential; dECM, decellularized extracellular matrix; PNM, peripheral nerve matrix; SFI, sciatic nerve function index.
As a natural material that can mimic the tissue microenvironment, decellularized matrices offer structures conducive to cell adhesion, mechanical properties supportive of tissue integration, and bioactivity-promoting neural regeneration. The primary limitations to their development and application lie in their fabrication and associated costs. Striking a balance between safety, immune modulation, and retention of active components during fabrication is crucial for developing hydrogels with translational significance.
2.2 Artificial-component hydrogels for PNI repair
2.2.1 Polyvinyl alcohol (PVA)
PVA is widely used as a tissue engineering material to prepare eye drops, artificial joints, and tissue barriers. However, its application is limited by its low mechanical stress threshold due to traditional physical cross-linkage, low purity, and inhomogeneous cross-linking caused by chemical cross-linkages.127-129 Oxidative modification, double cross-linkages, and multi-walled carbon nanotube implantations are common methods to improve its performance.61, 130 Stocco et al.130 used 1% oxidant-treated PVA to develop PNI repair cannulas with improved elasticity and suture resistance. These cannulas were shown to maintain their shapes during implantation and promoted regeneration of axons at the injury site, allowing the formation of myelinated nerve fibers similar to the original nerve size. Porzionato et al.131 suggested that nerve cannulas prepared from oxidized PVA were more conducive to releasing ciliary neurotrophic factors than unoxidized PVA. Additionally, Aregueta-Robles et al.132 found that cross-linked PVA with biomacromolecule silk glue and gelatin improved its recruitment of and adhesion to SCs. Recently, hydrogels with multi-walled carbon nanotubes doped with PVA were confirmed to have better ductility, electrical conductivity, and enhanced self-healing ability after injury.133-135 However, further investigations into the application of these hydrogels in PNI repair are necessary, especially regarding its regulation of the inflammatory response of damaged nerves and the induction and regulation of cells such as SCs.
PVA hydrogels demonstrate limitations in mechanical properties, degradability, and support for cell adhesion.136 However, oxidative modifications provide controlled tuning of PVA's mechanical and degradable characteristics while blending with other materials, which can modify its mechanical properties and cell adhesion potential.137, 138 These approaches present potential directions for the development of hydrogels for neural repair. Consequently, enhancing the biocompatibility of composite hydrogels containing PVA is imperative.
2.2.2 Polyethylene glycol (PEG)
Compared to PVA, PEG and its derivatives are the most widely used synthetic biocompatible hydrogel materials. PEG promotes cell rearrangements with its highly spreadable and tractable structures during degradation,139 which provides the theoretical basis for PEG hydrogels's ability to fill along nerve alignments during injury repair. PEG hydrogels are widely used in PNI repair owing to their convenient preparation processes, relatively low costs, simple chemical modifications, and easily tunable mechanical properties.61 Studies have confirmed that the fusion of allogeneic peripheral nerve grafts with PEG can reduce the immune response, significantly inhibit Wallerian degeneration, rapidly re-establish axonal conduction, re-innervate the injured nerve to the target organ, and even restore body functioning to its original level.33, 140 Unfortunately, original PEG hydrogels and their derivatives cannot support cell adhesion due to their biological inertness. Hence, modification through cross-linkages is needed to improve their properties and enhance their bioactivity to allow cell adhesion.61 PEG hydrogels conjugated with integrin-binding motifs such as RGD or MMPs are more conducive to multicellular aggregation in larger volumes.141 Based on these findings, PEG hydrogels and their derivatives will be better applied in PNI repair after eliminating these defects.
2.2.3 Polycaprolactone (PCL)
PCL has been widely used in PNI repair for controlled drug release, cell carriage, and mechanical support amongst other applications and targeted enhancement of its mechanical properties may be the focus of future research.61 Niu et al.142 prepared a PNI-repair scaffold cross-linked with PCL and PEG, which increased its surface area and enhanced its cell adhesion capacity. Various PCL materials loaded with urolithin-A,143 encapsulated with magnesium ions,144 and modified with type I collagen and mineralized collagen,145 have been prepared and examined. These conduits play an important role in recruiting SCs and guiding nerve fiber regeneration while effectively maintaining drug concentrations and efficacy.
PCL is extensively used in synthetic hydrogels for PNI repair due to its clinically relevant mechanical strength, inherent softness, ease of fabrication, prolonged biodegradation cycles, and favorable biocompatibility.146 However, it exhibits inferior hydrophilicity compared to PVA, which could influence its interactions with cells and biomolecules. Surface modification has emerged as an effective strategy to address this limitation. Therefore, strategies such as PCL surface modification to create structures conducive to cell adhesion are likely to be the primary directions for developing PCL hydrogels for neural repair in the future.147
2.3 Special-component hydrogels for PNI repair
2.3.1 DNA
The most important feature of DNA hydrogels is that they can carry a large amount of inherited information.92, 148 This may have positive implications for regulating cell distribution, genetics, and metabolism at the implantation site. However, these hydrogels have disadvantages such as difficult and expensive preparation costs, susceptibility to external stimuli such as environmental changes that may modify genetic information and physiological functions, and undefined safety when producing regulatory responses in organisms.61 Liu et al.124 developed a novel delivery system composed of DNA hydrogels, VEGFs, and NGFs. They exploited the difference in the degradation rates of X- and T-type DNA to achieve the bidirectional release of VEGFs and NGFs, which promoted the repair of PNIs. Presently, most DNA hydrogels are used for drug delivery. Although research on the preparation and practical application of DNA hydrogels remains immature, it has received considerable attention for its unique molecular recognition, ability to protect biologically active, functional proteins, and modifiability based on multiple recognition sites.149 In future studies, it will be necessary to investigate the development and preparation of DNA hydrogels from the perspective of molecular recognition and protection. Moreover, further research should focus on the regulatory mechanisms, preparation costs, and degradation rate of DNA in vivo. The application of DNA hydrogels as direct filler materials or cell carriers should also be explored. In summary, the mechanical properties of DNA hydrogels are inferior, even when compared to other hydrogels with suboptimal mechanical attributes, such as those based on gelatin or collagen. Moreover, their compromised stability, susceptibility to enzymatic degradation in vivo, and high fabrication and purification costs have constrained their broader applications. Incorporating other materials to enhance their stability and mechanical strength could be a direction for future improvements. Given DNA's nanoscale programmability, which facilitates superior cellular and molecular interactions, hydrogels with a DNA component have promising potential in tissue regeneration fields, including neural repair.150, 151
In general, the biological activity of natural hydrogels is better than artificial ones. However, artificial hydrogels have unique advantages due to their controllable characterization, such as the mechanical strength and viscosity of the material (details are listed in Table 3 62, 64, 65, 69, 72-80). The specific conditions of the materials may also be affected by other conditions, such as temperature, pH, treatment method, etc.
3 THE APPLICATION OF COMPOSITE, FUNCTIONALIZED HYDROGELS IN PNI REPAIR
In tissue engineering for PNI repair, the demand to develop bioactive scaffolds similar to natural nerve tissues to maintain, restore, or improve the function of damaged nerve tissues is urgent.152 Hollow nerve cannulas prepared from hydrogels can connect the proximal and distal ends of severed nerves and target the secretion of protein gels from the severed ends of injured nerves. They can also regulate the secretion of non-neural and neural cells to generate ECM components that migrate to or reorganize new matrixes.153 Although the effects of ECM components on repairing small-gap PNIs (3–10 mm) in this way have been clinically shown, insufficient formation of ECM components in the tissue cells at the point of injury prevents the migration of SCs to the defect site in instances of larger nerve defects.34 Therefore, using hydrogels as a biological factor delivery system, modifying original hydrogels, and encapsulating cells within hydrogels may be essential for repairing PNIs.
3.1 Composite, functionalized hydrogels in PNI repair
Given the state of past and current research, hydrogels' functionalization, property enhancement, and shape adjustment are currently the main directions in PNI repair efforts.
Improving the performance of composite hydrogels through the transformation and modification of basic materials is an important method for improving hydrogel materials. One approach for property modification is the transformation of hydrogels from conventional 2D to 3D structures. Chen et al.154 synthesized PGG1S hydrogel catheters via in situ, free radical polymerization based on polyacrylamide, graphene oxide, gelatin, and sodium alginate. They verified the superiority of these catheters in characterization, swelling behavior, tensile properties, and cell adhesion ability. Moreover, the catheters were found to promote SC proliferation and enhance PNI repair.
Functionalization is another direction for hydrogel development. By using polysaccharide chitin, Huang et al.155 modified hydrogels with poly(3,4-ethylenedioxythiophene) (PEDOT) nanoparticles to improve the electrostatic interaction between chitin and negatively charged PEDOT through the partial deacetylation of chitin to expose the amino group. This enhanced the strength of the composite hydrogels. The researchers adopted the cell adhesion tetrapeptide cys—arg—gli—asp (CRGD) (ht -PEDOT-p) to further modify the hydrogel membrane to induce damaged sciatic nerve regeneration with improved bioactivity and motified binding sites. This may enhance PNI repair by promoting the adhesion and proliferation of SCs and angiogenesis. Increasing the electrical conductivity of hydrogels and enhancing nerve signaling are also effective methods to obtain functionalized hydrogels. Dong et al.59 developed a photo-stimulation-responsive, highly stretchable, conductive polymer hydrogel. Its conductivity was similar to that of sciatic nerves. It also retained mechanical ductility and high durability, as confirmed by electromyography and current measurements. This hydrogel could also up-regulate the expression of the astrocyte-specific glial fibrillary acidic protein at the severed nerve end and modulate the calcitonin gene-related peptide level, so they are similar to those in a normal sciatic nerve. These advantages make the hydrogel a candidate material in PNI repair. He et al.156 recently prepared a hybrid hydrogel with injectability and electrical conductivity by homogeneously incorporating pristine carbon nanotubes into functional, self-assembled peptides while retaining the basic advantages of injectable hydrogels. The experimental results showed that electrical stimulation passed through the hydrogel, which promoted axon growth and migration of SCs from the DRGs to the injury site, enhanced the interaction between SCs and axons, and improved myelin formation. Hu et al.157 combined conductive materials and trophic factors with cannula structures to develop a nerve repair cannula in the form of reduced graphene oxide nanosheets and modified gelatin methacrylate hydrogel encapsulated with BDNF. This cannula had an excellent topological structure and performed well when repairing a 10 mm sciatic nerve defect in rats.
Grooved structures are more effective in promoting axonal growth than smooth ones, effectively reducing axonal dispersion and increasing axonal length.158 As matrix elasticity and topographic guidance are key factors regulating tissue regeneration, polyacrylamide/chitosan (PAM/CS) composite hydrogels with synergistic elastic and morphological properties were prepared by Liu et al. using in situ free radicals.158 In vitro experiments have shown that DRGs had substantial growth ability and improved orientation on the PAM/CS composite hydrogel with an elastic modulus of 5.822 kPa/8.41 kPa and a surface groove width of 30 μm. This suggests that different hydrogel shapes and structures (e.g., convolutions, furrows, folds, etc.) may affect PNI repair. However, it is necessary to explore the synergistic effect of matrix elasticity and topography guidance, the regulation of the pathway mechanism, the specific process of signal transduction, and the degree of cellular molecular involvement.
The targeted functionalization of hydrogels can be realized by (1) enhancing their conductivity, mechanical properties, etc., (2) modifying basic materials to improve their biological functions, and (3) changing their overall structure to generate 3D structures or enhance their topography-guided capabilities. Hydrogels modified with these methods may exhibit favorable results in guiding axons between the proximal and distal ends of damaged peripheral nerves. They may also effectively promote cell proliferation, biological factor secretion, and the expression of related repair genes. However, misdirected, overlapping, or dispersion of axonal growth should be avoided when modifying hydrogels. Moreover, since nerve regeneration in vivo (especially the directed growth of axons) is a complex process directed by the combination of topographical, electrical, chemotactic, and tactile signals, the degradation, cytotoxicity, immunogenicity, and long-term effects of these hydrogels in vivo and their complex effects on original physiological processes need to be verified.
3.2 Biological-factor-delivery hydrogels in PNI repair
A variety of biological factors are relevant to the repair PNIs. In addition to conventional direct-acting factors, BDNFs, cerebral dopamine neurotrophic factors, and VEGFs have also been widely studied for their anti-inflammatory effects in the early stages of PNIs. Based on the promotion of angiogenic properties during the construction of bioactive materials for neural tissue engineering, Xu et al.159 developed an injectable hydrogel encapsulated with a VEGF mimetic peptide (QK), which achieved a sustainable release of VEGFs. In vivo experimental results showed that the hydrogel promoted the revascularization and polarization of M2-type macrophages, which provided favorable conditions for PNI repair.
We hypothesized that the synergistic effect of multiple biological factors achieved by a hydrogel-based bio-scaffold would amplify the impact of the material on PNI repair. The synergistic effects of the VEGF-mimetic peptide RGI and the BDNF-mimetic peptide KLT promote the repair of PNI, which was demonstrated in a large-segment sciatic nerve defect model (15 mm).160 Rao et al.160 combined integrated RGI/KLT with a CS hydrogel and applied it to a PNI site. The material promoted vascular penetration, proliferation of SCs, and the secretion of neurotrophic factors. Unfortunately, the study did not identify the specific mechanism for the synergistic effect of RGI and KLT or the optimal dose needed to achieve this effect. The preparation of novel hydrogels in this way requires a structural analysis of the biological factors, the preparation of mimetic peptides, and the selection of suitable hydrogel materials and cross-linking methods. According to several similar studies, the number of intraluminal fibers for these hydrogel cannulas should be adjusted and optimized according to different injury sites, sizes, and gel structures to prevent axonal regeneration blockage and the migration of non-neuronal cells caused by overly dense intraluminal matrixes.
Other biological factors, such as Wnt5a, can promote PNI repair. As a key downstream protein of the NGF signaling pathway, Wnt5a can promote an axon's growth, differentiation, branching, and extension via activating protein kinase c and calpain.161-163 Liu et al.164 prepared a fibronectin-assembled hydrogel with a nontoxic cross-linker to carry Wnt5a for PNI repair. Their results suggest that the hydrogel could recruit SCs, promote their proliferation, and enhance the release of VEGFs and NGFs. The hydrogel also had a synergistic effect on promoting angiogenesis and nerve regeneration. The integration and filling of other ECMs into the hydrogel also had positive effects on PNI repair. Gonzalez-Perez et al.165 added various ECM components, including laminin and fibronectin, into a prepared CS hydrogel. Their results indicated that the mixed combination of collagen and fibronectin was more effective, with the stable combination potentially improving the regeneration quality of large segment defects.
Hydrogels could prevent bioprotein factors from degradation and amplify and enhance the effects of biological factors. Therefore, their integration with different factors has received considerable attention. For example, acidic fibroblast growth factors (aFGFs) can promote neuronal growth and survival and have been regarded as a possible therapeutic approach for PNI repair.166 However, the short half-life and instability of aFGFs in vivo limit their practical therapeutic utility.167 To improve the biological activity of aFGFs, Liu et al.167 prepared a hydrogel with heparin-like properties and loaded aFGFs into it for injection. The aFGFs were released with the breakdown of the hydrogel, which had a protective effect against RSC96 cell injury and promoted sciatic nerve repair in injured rats. However, the mechanism underlying how aFGFs affect sciatic nerve repair has not been fully clarified. Hence, more studies are needed to confirm the current observations and elucidate the mechanisms of action of these novel hydrogels. Such efforts may provide new ideas for researching and developing more hydrogels with excellent performance.
Based on the results of recent studies, a key point when developing hydrogels with biological factors is ensuring the overall biosafety of the material in addition to choosing the appropriate cross-linking method to maintain stability and possibly play a synergistic role (see Table 4 168-173 for a summary of some of these studies). The selection of biological factors (e.g., adhesion factors) is equally important in developing new hydrogels. The effects of the biological factor, its half-life, how it binds to the hydrogel, and its controlled release are all variables to be considered.174, 175 In an attempt to clarify these issues, it is necessary to: (1) investigate biological factors that can promote nerve repair to reveal their shortcomings and investigate ways to compensate for the disadvantages by combining them with hydrogels; (2) explore basic materials, modify materials for preparing hydrogels, and enhance the properties of hydrogels by combining the popular concepts of targeted modification, peptide self-assembly, nano, and exosomes to achieve targeted recognition and biological release; and, (3) conduct clinical translational research and obtain human research data to promote hydrogels' further translations and clinical applications.
| Name of hydrogels | Main components | Delivery/Modified components | Experimental model | Mechanism of action | Improvement by hydrogels | References |
|---|---|---|---|---|---|---|
| Chitosan/polycaprolactone conduits hydrogel | Chitosan and polycaprolactone | Nerve growth factor | mHippoE-18 embryonic hippocampal cells | Stimulates axonal growth and supports monocyte activation | Controls the spatiotemporal release of nerve growth factors to support regrowing nerves over a prolonged period | [168] |
| NGF/PLCL/collagen/hyaluronan hydrogel | Poly-L-lactide-co-caprolactone, collagen, and hyaluronan | Nerve growth factor | Rat model | Significantly increases neurite extensions from dorsal root ganglia explants | Enhances sensory neurite outgrowths in vitro and sensory recovery in vivo | [169] |
| GelMA/PEtOx hydrogel | Gelatin methacrylate/poly(2-ethy-2-oxazoline) | 4-Aminopyridine | Rat model | Blocks potassium channels to increase neuromuscular function | Bridges the nerve and controls the release of 4-AP to maintain its concentration | [170] |
| Let-7a antagomir control-released chitosan-hydrogel | Chitosan-hydrogel | Let-7a antagomir | Rat model | Targets and regulates nerve growth factors to control peripheral nerve regeneration | Effectively achieves controlled, localized, and sustained delivery of let-7a antagomir by integrating it into hydrogels | [171] |
| HIF-1α/Pluronic F-127 hydrogel | Pluronic F-127 | HIF-1α | Rat model | Modulates inflammation caused by injury, promotes neovascularization progress, and protects neurons | Effectively promotes spinal root regeneration and functional recovery post-brachial plexus avulsion | [172] |
| Berberine/Alginate/Chitosan hydrogel | Alginate/chitosan | Berberine | Rat model | Berberine has dose-dependent effects on cell proliferation to promote SC proliferation and prevent inflammation | Berberine, especially 1% hydrogel containing berberine, can promote the regeneration of sciatic nerves | [173] |
3.3 Cell-carrying hydrogels promote PNI repair
Recent studies have demonstrated that GMSCs have multidirectional differentiation capabilities and potent immunomodulatory/anti-inflammatory functions. In addition, they have exhibited therapeutic potential in animal models of various human diseases.176, 177 In some early studies, 3D-collagen hydrogels were used to mediate the differentiation of GMSCs into Schwann-like cells (GiSCs). These results demonstrated the feasibility of constructing functionalized, cell-laden neural guidance conduits.101 Zhang et al.178 (Figure 2) used methacrylate-based 3D-collagen hydrogels to encapsulate GMSCs. Their results demonstrated that this process resulted in the up-regulated expression of SC markers and neurotrophic factors in cells and differentiated GMSCs into GiSCs.
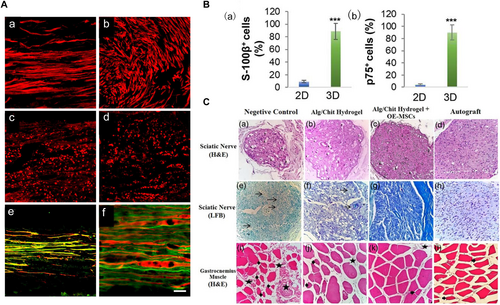
The potential of hydrogels carrying various cell types in accelerating the regeneration of peripheral nerves at the cellular and tissue levels. (A) Compared to hollow conduits (b) and standard hydrogels (d), the 3D hydrogel structures (a, c) significantly enhanced the orderly and uniform alignment of Schwann cells, indicated in red. Scale bar: 62.5 μm (a–d), 100 μm (e) and 25 μm (f). (B) Immunofluorescence results demonstrated that, relative to two-dimensional hydrogels, three-dimensional hydrogels substantially bolstered the differentiation of gingiva-derived mesenchymal stem cells into Schwann cells and increased the secretion of neurotrophic factors. (C) Following validation of a rat sciatic nerve deficit model, hydrogel scaffolds laden with readily accessible olfactory ectomesenchymal stem cells exhibited therapeutic outcomes comparable to autologous transplantation. Arrowheads: vacuolation, star: collagen hyperplasia, thick arrows: atrophied muscle fiber, thin arrows: edema; muscle: ×400, nerve: ×200. Panels reproduced with permission from (A)179, Elsevier; (B)178, Authors; (C)180, Wiley. MSCs, mesenchymal stem cells.
GiSCs encapsulated in 3D collagen hydrogels also had an effective immunosuppressive impact on the activation of pro-inflammatory M1 macrophages while promoting the polarization of anti-inflammatory M2 macrophages. These studies have shown the important role of hydrogels loaded with GiSCs in the repair of PNIs, and based on the study by Zhang et al., it can be assumed that cell differentiation induced by neurotrophic, phenotypic, and immune-related mechanisms plays an essential role in this process under the environmental stimulation provided by the hydrogel and the damaged nerve. Finally, olfactory mucosa-derived stem cells have high accessibility and proliferation compared with GMSCs. Salehi et al.180 used sodium alginate/CS hydrogels to carry olfactory exogenous MSCs and demonstrated their effects on PNI repair.
The role of adipose-derived stem cells (ASCs) in PNI repair has also been highlighted. ASCs are different from other hydrogel materials that conventionally carry stem cells. Co-carrying ASCs with relevant neurotrophic drugs (or treating them with neurotrophic drugs before hydrogel carriage) has been a research strategy in recent years.181 Prautsch et al.182 conducted an in vitro stimulation of ASCs with NGFs or VEGFs. The prior stimulation and use of fibrin-hydrogel nerve catheters to assist ASC delivery via intramural and intraluminal loading modes may promote early regeneration of peripheral nerves by increasing the number of β-tubulin III+ axons. The use of hydrogels would therefore enhance the therapeutic effect of ASCs compared to conventional injection protocols. Another study combined a fibrin, gel-based, tacrolimus extended-release system with rat ASCs. The results indicated that the combined application could control the release of 100 ng/mL of tacrolimus under the encapsulated buffer of protein gels without cytotoxic effects on MSCs. Moreover, it may promote regeneration after a PNI.183 Based on the widely verified treatment of PNIs with MSCs, Han et al.184 proposed a controllable 3D-hydrogel external hybrid microneedles array patch to achieve in situ repair of injured spinal cords to solve the problems of MSC stemness loss caused by traditional 2D culture and the resultant decrease in the therapeutic effect of exosomes. Three-dimensional culturing of MSCs can maintain their stemness and produce 3D exosomes that effectively alleviate inflammation and glial scarring. These continuous explorations provide new ideas for the repair of PNIs.
However, ethical issues limit the clinical translation of cell-carrying hydrogel materials. In addition (and compared with hydrogels directly modified and loaded with biological factors), cells as complex organic monoliths impose higher safety requirements during hydrogel preparation. When using co-loading systems of biological factors and stem cells, attention must be paid to the interactions between them. Some of these cell-carrying hydrogels for PNI repair are summarized in Table 5,179, 185-188 which may provide a more comprehensive and systematic understanding of these hydrogels.
| Name of hydrogels | Main components | Loaded cells | Experimental model | Mechanism of action | Improvement by hydrogels | References |
|---|---|---|---|---|---|---|
| Poly (2-hydroxyethylmethacrylate) hydrogels | Poly (2-hydroxyethylmethacrylate) and collagen IV-impregnated | SCs | Rat model | Preferentially supports transplanted SCs, promotes the generation of RT97 axons, and myelinates them with SCs | Modifies the host glial reaction and supports the survival of transplanted SCs | [185] |
| BD™ PuraMatrix™ peptide hydrogel | Peptide | SCs | Rat model | Considerably increases the regeneration distance with axons crossing the injury gap | Increases the rate of axonal regeneration across a nerve defect | [179] |
| Chitosan-collagen hydrogel | Chitosan-collagen | Neural stem cells and SCs | Rat model | Induces the extension of axons targeting the hydrogel conduit | Promotes the high-quality regeneration of peripheral nerves via specific orientations | [186] |
| ECH-Exos hydrogel | Mixture of TA, Py, and Fe3+ combined with electroconductive hydrogels | BMSCs-Exos | Rat model | Promotes SC attachment and migration–with the capacity to modulate polarization from M1 to M2 macrophages through the NF-κB pathway | Promotes myelin-associated axonal regeneration and attenuates inflammatory pain | [187] |
| ASC/PCL hydrogel | Polycaprolactone and poloxamer | Adipose stem cells | Rat model | The overall distribution of CSA in muscle fibers in the contralateral TA muscle of the uninjured limb is reconstructed and can promote the proliferation and recruitment of SCs | Significant benefits of inclusion of ASCs to the rate and magnitude of peripheral nerve regeneration and functional recovery of muscle contraction to levels equivalent to autograft implantation | [188] |
- Abbreviation: CSA, cross-sectional area.
4 SUMMARY AND OUTLOOK
Despite recent advances in materials science, PNI repair remains a challenge for clinicians. Currently, poor clinical outcomes are caused by the low regenerative efficiency of peripheral nerves and the complexity of the healing process. Hydrogel scaffolding materials that use a well-combined triad of living cells, biological factors, and scaffolds composed of each substrate may overcome the limitations of current PNI treatment strategies. Many animal models have been constructed to verify the functions of biomaterials (Table 6). A suitable biomaterial for neural regeneration should have biocompatibility, hemocompatibility, immunocompatibility, porosity, directed axonal growth, suitable mechanical properties, long-term stability, and durability during implantation. Given these needs, hydrogels have received considerable attention from researchers due to their superior physicochemical and biological properties. Moreover, these favorable properties enable hydrogels to be used as tissue engineering materials in PNI repair. The mechanical properties of hydrogels are similar to nerve tissue, and their swelling properties provide a contracted 3D structure that accelerates nerve growth. The mechanical strength of modified hydrogels is also sufficient to maintain the morphology, proliferation, and differentiation of nerve-associated cells. Coupled with better biocompatibility, negligible immunogenicity, and higher similarity to the ECM of neural tissues, modified hydrogels have more extensive applications than unmodified ones. The use of rapid prototyping technologies (e.g., biomapping and 3D printing) allows the precise construction of materials with established structures and details closest to the original nerve tissue, which promotes the development and application of novel hydrogels that combine loaded growth factors and cells to further control intra- and extracellular signaling mechanisms and stimuli, thus contributing to PNI repair. However, the clinical translation of hydrogels in peripheral nerve repair faces some challenges. Examples include complex tissue environments, precise drug release control, the feasibility of surgical implantation, individual differences, and clinical monitoring and evaluation methods following implantation. If these issues are surmounted, more in-depth research and validation can occur, with collaboration between professionals in multiple fields, such as material scientists, biologists, and surgeons, as a critical part of this process.
| Animal | Strain | Gender/Weight | Surgical procedure | Reference |
|---|---|---|---|---|
| Mouse | Swiss Webster | Male | Proximal and distal nerve stumps of the transected sciatic nerve were aligned and sutured 1 mm into 5-mm inert silicone conduits with a 10–0 suture to create a short gap defect (3 mm) for investigation of the early (5 days) host macrophage response to PNM | [189] |
| Mouse | Swiss-Webster | / | The tibial nerve was transected 5 mm proximal to its insertion into the gastrocnemius | [190] |
| Rats | Lewis | Male/250–275 g | A 10-mm segment of the nerve was excised, and an appropriate 12-mm-long tube was sutured into the resulting gap. To create a gap of 10 mm, the proximal and distal nerve stumps were inserted into the inner lumen of the tube at a distance of 1 mm | [191] |
| Rats | Sprague Dawley | Female/250∼300 g | The nerve was carefully dissected free of the surrounding tissue, and a 10 mm nerve segment was excised with scissors. The defect was repaired with a 12 mm silicone tube | [84] |
| Rabbits | New Zealand White rabbits | Male/3.5–4 kg | A 2 or 3 cm defect was generated, allowing the proximal and distal ends to retract | [192] |
| Dogs | Beagle | Male/12–15 kg | A sciatic nerve segment was excised to create a 50-mm long defect after retraction of the nerve ends | [193] |
- Abbreviation: PNM, peripheral nerve matrix.
-
Native nerve axons are affected by circulating electrical impulses for information transmission, and the application of conductive materials or appropriate electrical stimulation is beneficial for promoting the survival and lengthening of axons.
-
Neovascularization can ensure cell survival by transporting nutrients and oxygen, supporting cell metabolism, and avoiding necrosis. Encapsulating growth factors and cells associated with angiogenesis to form a vascular network around the injured peripheral nerve may facilitate PNI repair.
-
The biochemical-molecular regulation of cell-ECM interactions (e.g., NGFs) and cell-cell interactions (e.g., calmodulin and neural CAMs) is a focus for relevant future research.
-
The signal transduction and cell growth environment of mechanical stimuli under the influence of the elastic modulus and the surface and internal morphology of the biomaterials should be considered as major factors for the development of hydrogels. Their changes should be considered for hydrogel preparation.
-
Combining precisely targeted therapy with other therapeutic strategies (e.g., NGCs, drug delivery, and cell therapy) can improve peripheral nerve repair (Figure 3).
In conclusion, applying novel hydrogels as substrates and combining biological factors and cells to prepare peripheral nerve repair material results in excellent performance. As carriers, hydrogels are malleable and biocompatible, and can provide support and an environment that facilitates the release and targeted action of growth factors or cells. They can be engineered to have controlled-release properties that regulate the rate and timing of growth factor release. This helps to mimic physiological conditions and ensures that growth factors are released at the right time during the repair process. Alternatively, by adding a directional factor, such as a neural adhesion molecule or other bioactive molecule to the hydrogel, the directionality of the neurons can be improved and guided to grow, which contributes to the directional growth of nerve protrusions. Hydrogels also protect growth factors from degradation or premature inactivation, ensuring they work at the right time and place. Finally, their ability to provide a surface for cell adhesion aids in cell colonization and survival at the injury site, thereby promoting nerve repair. Investigating hydrogels' intrinsic biological mechanisms in PNI repair under novel stimulating factors is expected to provide comprehensive insights for clinicians when treating PNIs.
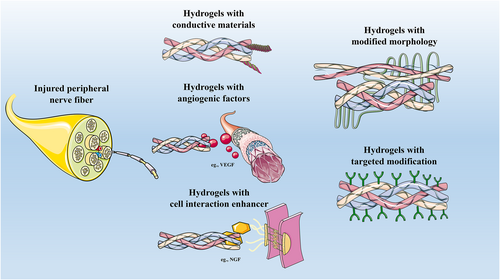
Various types of hydrogels in PNI repair. Generally, five types of hydrogels can repair injured peripheral nerve fibers: (1) Hydrogels with conductive materials; (2) Hydrogels with angiogenic factors; (3) Hydrogels with cell interaction enhancer; (4) Hydrogels with modified morphology; (5) Hydrogels with targeted modification. PNI, peripheral nerve injury.
AUTHOR CONTRIBUTIONS
Shicheng Jia: Investigation; visualization; writing—original draft. Hongfa Zhou: Investigation; data curation. Jiayou Chen: Validation; resources. Jiashan Lin: Methodology; validation; resources. Xinlei Zhu: Methodology; validation; resources. Jian Weng: Visualization. Wei Li: Conceptualization; methodology; resources. Fei Yu: Conceptualization; methodology; resources; supervision; writing—review & editing.
ACKNOWLEDGMENTS
This research was supported by grants from the National Natural Science Foundation of China (No. 82102568), Shenzhen Key Medical Discipline Construction Fund (No. SZXK023), Sanming Project of Medicine in Shenzhen (No. SZSM202211038), Basic and Applied Basic Research Foundation of Guangdong Province (No. 2022A1515220111) and The Scientific Research Foundation of Peking University Shenzhen Hospital (No. KYQD2021099). Graphical abstract image and Figure 3 are drawn by added text, markings, and annotations after ultization of elements from “Nervous system,” “Intracellular structure,” “Receptors,” “Heart and arteries,” and “Tisuue” from Websites of sections Servier Medical Art by Servier (https://smart.servier.com), licensed under a Creative Commons Attribution 3.0 Unported License.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
Ethics approval was not needed in this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




