Advances of therapy for Alzheimer's disease: An updated review
Can Mei, Jianbo Zhan and Shuzhen Zhu are equal contributions.
Abstract
Alzheimer's disease (AD) is a type of dementia characterized by a decline in brain function, which leads to the inability to perform activities independently. Many researchers recognize abnormalities related to beta-amyloid as the main cause of the disease (i.e., the beta-amyloid hypothesis), but aging, genetics, coronary heart disease, environmental factors, gender, and other risk factors may also contribute to AD development. Three drugs with different mechanisms are available for AD treatment: cholinesterase inhibitors, N-methyl d-aspartate, and aducanumab. This study reviewed the therapies that are already applied in clinical practice and those that are currently being investigated for clinical use. These therapies include not only pharmacological treatments but also non-pharmacological treatments, such as gut flora therapy and music therapy. A comprehensive understanding of these therapies is necessary to enable early intervention, improve patients' physical and mental conditions, delay the occurrence and development of AD, and extend patients' healthy lifespans.
Key points
What is already known about this topic?
-
At present, the treatment of Alzheimer's disease (AD) includes drug and non-drug therapies. Commonly used drugs include acetylcholinesterase inhibitors (such as Donepezil, levodopa) and NMDA receptor antagonists (such as Memantine), as well as aducanumab. Non-pharmacological treatments: Non-pharmacological treatments can improve the quality of life for people with Alzheimer's by fostering a healthy lifestyle and providing support.
What does this study add?
-
This paper describes the development of drugs for the treatment of AD, including acetylcholinesterase inhibitors, NMDA receptor antagonists and the latest drug aducanumab, which have been used for many years, Multi-target drugs, miR-485-3p antisense oligonucleotide, Gamma-secretase, Immunotherapy. The follow-up focuses on the description of non-drug treatment, such as Music Therapy, Intestinal flora, acupuncture, stem cell therapy, and nanoparticle technology treatment.
1 INTRODUCTION
Many factors can increase AD risk, such as age, genetics, underlying diseases, environmental factors, and so on (Figure 1).1 AD is the most common type of dementia in older adults and accounts for 50%–70% of dementia cases.2-4 In 2022, the number of older adults with AD reached 6.5 million, and this number is predicted to increase to 13.8 million by 2060. In the United States, AD is the fifth leading cause of death among Americans 65 and older.5 Furthermore, AD deaths have increased by at least 145% over the past 19 years.6
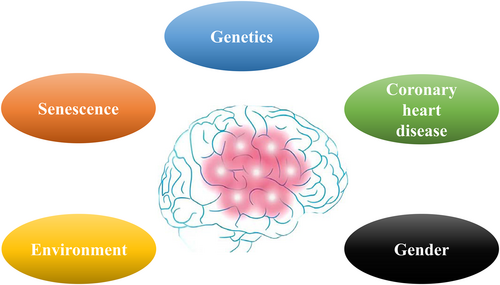
Brain imaging changes and influencing factors in Alzheimer's disease (AD).
AD not only places an immeasurable emotional burden on patients and their families but also represents a huge economic burden for society. In 2021, it was reported that people with AD and other types of dementia received approximately 16 billion hours of care from more than 11 million family members or other unpaid caregivers, with care costs reaching USD271.6 billion. A survey revealed that Americans do not have comprehensive knowledge of mild cognitive impairment (MCI), leading to persistent challenges for primary care physicians in diagnosing MCI.6
The pathogenesis of AD is unclear; the two major pathogenic mechanisms are recognized: amyloid β (Aβ) and Tau protein misfolding. Aβ deposition is associated with Tau accumulation,7 and Tau accumulation is associated with hypoglycemic metabolism, brain atrophy, and neurodegeneration.8 Several biological factors also drive protein aggregation, including apolipoprotein E4 allele (APOE4), neuro-inflammation, sleep disorders, and autophagic dysfunction.9 In addition, genetics, environmental factors, gender, underlying disease,10 oxidative stress, and cholinergic neuronal damage can lead to neurodegeneration.11 This study reviewed the existing diagnostic approaches for AD, which include not only pharmacological but also non-pharmacological treatments, such as music therapy, gut flora therapy, acupuncture, stem cell therapy, and nanoparticle therapy. In addition, this review explored potentially effective methods for the early identification and diagnosis of AD.
2 DIAGNOSTIC CRITERIA FOR ALZHEIMER’S DISEASE
Clinicians use various examinations to assess patients with suspected AD, including neurological examinations, neuronal magnetic resonance imaging (MRI), and laboratory tests (e.g., vitamin B12), in addition to the patient's medical and family history.12 In recent years, the diagnostic criteria for AD have been revised multiple times. In 2011, the National Institute on Aging-Alzheimer's Disease Association (NIA-AA) updated the criteria published in 1984 (the National Institute of Neurological and Communicative Diseases and Stroke/AD and Related Disorders Association, NINCDS-ADRDA, criteria) to improve the specificity and sensitivity of AD diagnosis. This update incorporated biomarkers into the clinical criteria for AD and specified their use in both clinical and research settings for diagnosing probable and possible AD with or without evidence of the AD pathos-physiological process. In 2018, the NIA-AA Working Group proposed a new set of diagnostic criteria for AD, the core characteristics of which were 1) abnormal clinical manifestations and cognitive tests; 2) biomarker evidence; and 3) structural or functional imaging evidence, which could further classify AD into two diagnostic subgroups-typical AD and atypical AD. Finally, in 2021, the AD International Working Group (IWG) updated its recommendations for AD diagnosis. The new recommendations, which focused on neuroimaging and the use of liquid biomarkers, emphasized the importance of early clinical diagnosis and divided AD into three stages: preclinical AD, AD in the MCI stage, and clinical AD. The IWG recommended using Aβ positron emission tomography (PET) and Tau PET scans to identify Aβ and Tau aggregates to distinguish normal aging from AD. The IWG recommended liquid biomarkers to diagnose and screen patients enrolled in clinical trials, where as they recommended blood biomarkers, such as serum neuro-filament light (NfL), to help assess the severity and prognosis of AD. In addition, the IWG highlighted the importance of individualized diagnosis and treatment to provide more accurate and effective treatments according to the patient's condition, bio-markers, and imaging findings.13
Several scales can also be used for the initial diagnosis of AD, such as the Mini-Mental State Examination (MMSE), the Hamilton Anxiety Inventory (HAMA),14 the Hamilton Depression Scale (HAMD),15 the Self-Esteem Scale (SES),16 the Satisfaction with Life Scale (SWLS),17 the Pittsburgh Sleep Quality Index (PSQI),18 and the Self-Rated Health Measurement Scale (SRHMS).19 All of these scales can reflect AD severity to some extent.
3 BIOMARKERS OF ALZHEIMER’S DISEASE
The early neuropathological features of AD can occur 15–20 years before disease onset.20 Although current treatments for AD are inadequate, it is feasible to slow its progression; furthermore, early and accurate diagnosis of AD could save up to USD 232.7 trillion in health and care costs.21 Two reliable AD biomarkers are currently available: (a) brain amyloid markers, as determined by PET and cerebrospinal fluid (CSF) analysis, and (b) neuronal damage markers, such as CSF Tau. To diagnose AD and evaluate its progress, fluorodeoxyglucose (FDG) PET can be used to evaluate metabolic activity, MRI can be used to measure brain atrophy,22 and electrochemical biosensing can be used to detect Tau-381 in human serum.23 In recent years, brain imaging and CSF testing have been used in clinical settings.24, 25 However, these methods are costly and invasive, which severely limits their use in mass screening.26, 27 Therefore, researchers have turned to inexpensive and minimally invasive peripheral blood biomarkers, such as Aβ1-42, T-Tau, and P-Tau in the peripheral blood, to screen for early AD. Our previous research compared the sensitivity, specificity, and area under the curve (AUC) of five proteins-SIRT1, IL-6, Aβ1-42, P-Tau, and T-Tau-in the peripheral blood. We found that the MMSE scores were above the cut-off value of 18, and the sensitivity, specificity, and AUC of SIRT1 proteins were much higher than those of the other four proteins. Therefore, the SIRT1 protein level can be used as an indicator to screen for earlyAD.28 In addition, plasma biomarkers, such as P-Tau181, NfL, and glial fibrillary acidic protein (GFAP), have recently been discovered. In another study, Aβ fragments of 40 and 42 amino acids were assessed using the most advanced single molecule array technique in blood samples from 1439 early and late-onset AD cases and 508 controls. P-Tau181, NfL, GFAP, and other biomarkers reached high prediction accuracy, with the area under the receiver operating characteristic curve (AUROC) reaching 0.81.29 In the latest study, the researchers studied the protein potential predictors of 85,934 patients and 401,577 normal European subjects, and created 1864 protein prediction models. ILT-4, PRPC, SHPS1, Siglec-3, SHPS1,Siglec-3,and Siglec-9 were particularly prominent, with Siglec-3 (called CD33) being reported as a risk factor for AD, with both mRNA levels and protein abundance increasing in AD patients compared to age-matched controls. This finding is consistent with our current study (Z = 4.47, p = 7.78 × 10 − 6).30 In another study, researchers evaluated 39,106 clinical cases of AD, 46,828 associated dementia cases, and 401,577 control cases, identifying 14 associated metabolites associated with AD risk. Only 3- (3-hydroxyphenyl) propionate content was positively correlated with AD. Other metabolites such as X-17178, 5alpha-androstan-3beta, 17betadiol disulfate were negatively correlated. Five microbiome features were further identified to be potentially related to the associations of five of the metabolites. Our study provides new insights into the etiology of AD that involves blood metabolites and gut microbiome, which warrants further investigation.31 (Table 1: AD treatment and diagnosis timeline).
| Time(Year) | Treatment method | Diagnostic method |
|---|---|---|
| 1906 | Alzheimer first reported case of AD | -- |
| 1970 | Alkaline phosphatase staining is used to improve diagnosis based on cytology | -- |
| 1984 | Successful isolation of beta-amyloid protein | -- |
| 1987 | The FDA approved tacrine as the first AD drug | Clinical diagnosis was made using symptom and cognitive impairment tests |
| 1993 | Clinical diagnostic criteria were developed by NINCDS-ADRDA | -- |
| 1996 | Donepezil is approved by the FDA | -- |
| 1998 | Histological examination confirmed APOE epsilon 4 as a risk factor for AD | -- |
| 2000 | Memantine is approved by the FDA | -- |
| 2007 | PIB-PET technology has been used in humans for the first time | Amyloid deposition was tracked using carbon-11 labeled PIB |
| 2010 | Amyvid granules (pittsburgh compound B) are approved by the FDA | A florbetapir F-18 probe from the university of Chicago was used to bind PET to detect beta-amyloid |
| 2011 | Florbetaben F-18 granules (PET developer) and T2 magnetic ResonanceImaging (T2-MRI) technology are approved by the FDA | Florbetaben + PET is used to detect beta-amyloid and T2-MRI is used to detect brain atrophy in Alzheimer's disease |
| 2019 | FDA approves aducanumab | -- |
| 2023 | The FDA approved leqembi for marketing | -- |
4 DRUG TREATMENT
Aβ can arise from abnormal processing of Aβ precursor protein (APP) and abnormal cleavage of APP by β- and γ-secretase.32, 33 However, Aβ triggers a cascade reaction that causes synaptic damage and neuronal loss, which are responsible for the pathological features of AD (i.e., neurofibrillary tangles (NFTs) composed of Aβ plaques and hyperphosphorylated Tau proteins), ultimately leading to neuro-degeneration.34
4.1 Traditional drug therapy
The first drug for the treatment of AD was approved in the United States on June 7, 2003.35 Before June 7, 2021, only two classes of drugs were approved for use: cholinesterase inhibitors (AChEIs, naturally derived, synthetic, and hybrid analogs), and antagonists of N-methyl-D-aspartate (NMDA). Currently, the following drugs are commonly used: donepezil (A, Figure 2), rivastigmine (B, Figure 2), galantamine (C, Figure 2), and memantine (D, Figure 2).36, 37 AchEIs reduce choline transmission, synaptic damage, and acetylcholine (ACh) production in the brains of patients with AD by blocking the breakdown of ACh by cholinesterase.38, 39 NMDA receptor antagonists inhibit the excessive activation of NMDA receptors, reduce the Ca2+ concentration, promote cell death and synaptic dysfunction, and restore normal organismal activity.1 However, these drugs can only alleviate the symptoms of AD and cannot prevent or cure the disease.40 As AD progresses, the drug dose must be increased, thus increasing the likelihood of secondary adverse effects.41, 42 On June 7, 2021, the US Food and Drug Administration (FDA)approved the marketing of adu-canumab, the first drug to act directly on the pathogenesis of AD rather than targeting the symptoms.43 Aducanumab can cross the blood-brain barrier (BBB) and selectively binds to Aβ plaques in the brain. Aducanumab has been shown to bind to Aβ amino acids 3-7 in a linear epitope that can distinguish between Aβ monomers, oligomers, and fibrillary aggregates44; this allows it to specifically target aggregated Aβ and reduces Aβ plaques in the brain. However, the efficacy and safety of aducanumab are still not guaranteed. Figure 3 shows the methods of aducanumab administration.
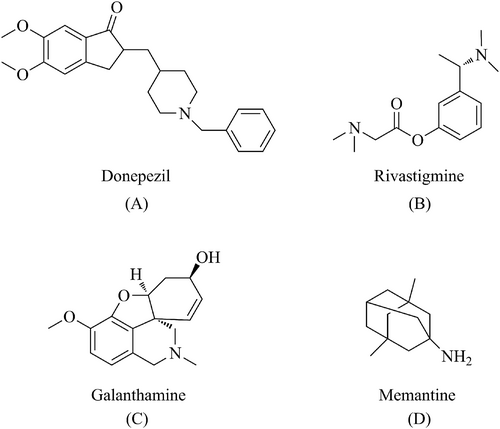
Drugs that have been approved for symptom alleviation in AD: donepezil (A), rivastigmine (B), galanthamine (C), and memantine (D).
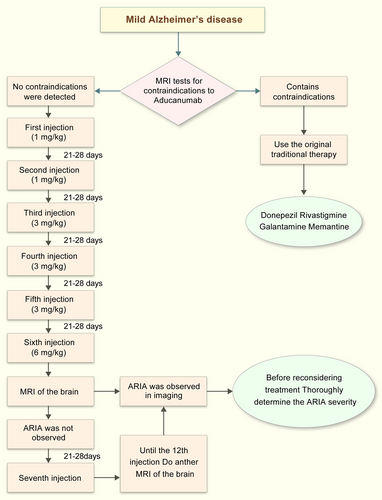
Methods for aducanumab use.
On January 6, 2023, the FDA accelerated the approval of Leqembi, a drug jointly developed by Eisai and Biogen. Leqembi is the second treatment that targets the underlying pathophysiology of AD. By encouraging the immune system to clear amyloid plaques, Leqembi may help slow or stop the progression of the disease, thereby promoting the cognitive and functional abilities of patients with AD.45 FDA-based phase 2 data showed a reduction in Aβ plaques in patients treated with Leqembi. Study 201 was a multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-exploring study of 856 patients with MCI due to AD or mild AD. The results showed a dose-and time-dependent reduction in Aβ plaques in treated patients; the Leqembi group received10 mg/kg every 2 weeks, and showed a signify-cant reduction in brain Aβ plaques from baseline to week 79 compared with the placebo group, which did show a reduction in Aβ plaques.46
Clarity AD is a global phase 3 trial comparing a Leqembit placebo in 1795 patients with early stage AD. Participants were randomly assigned to two groups according to clinical characteristics and regions. The primary outcome measures were cognitive and functional changes after 18 months, and the secondary outcomes were brain plaque changes and other cognitive and functional scales after 18 months. The results of the study, which were presented simultaneously at the 2022 CTAD meeting and in the New England Journal of Medicine, showed that Leqembi significantly reduced cognitive decline in patients with early stage AD, with a 27% reduction in clinical dementia rating-sum of boxes (CDR-SB) scores compared with the placebo group.46
4.2 Drug development
Although the above two classes of drugs can relieve some of the symptoms of patients with AD, their efficacy is unclear. Therefore, emerging drugs as well as medication methods are constantly being introduced.
4.2.1 Multi-target drugs
Although the pathogenesis of AD is not yet understood, it is associated with multiple responses, including a lack of cholinergic neurotransmission, defective Aβ metabolism (Aβ aggregation), Tau deposition and phosphorylation (forming NFTs), and inflammatory and oxidative pathways.47 Although currently approved drugs are limited by their single mode of action, multi-target drugs (MTDs) have recently emerged.48
For example, 5-hydroxytryptamine (A, Figure 4) can modulate multiple neuro-transmitter levels simultaneously. Although 5-hydroxytryptamine has shown promising results in animal models, its efficacy in humans remains unclear. Merck and Co. stopped a phase 3 human clinical trial on a 5-hydroxytryptamine-2A, because the expected results were not observed; the drug did not significantly slow AD-related cognitive decline.49 Other examples of drugs in this class are ladostigil [(N-propargyl- (3R) aminoindan-5yl)-ethyl methyl carbamate] (B, Figure 4),50 rosiglitazone (C, Figure 4), hydroxychloroquine (D, Figure 4), and miconazole (E, Figure 4), which are used in combination.51
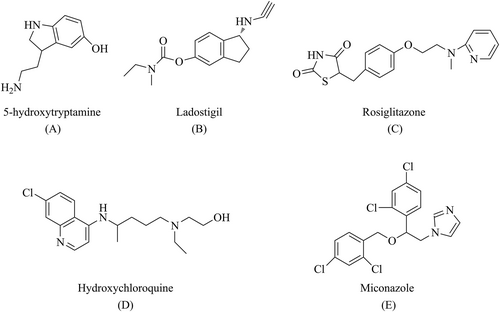
Common multi-target drugs (MTDs): p-5-hydroxytryptamine (A), ladostigil (B), rosiglitazone (C), hydroxychloroquine (D), and miconazole (E).
Although they are a promising treatment method, no MTDs have been approved for AD treatment. However, as of March 4, 2021, 111 clinical studies evaluating the efficacy and safety of MTD were in phase 2/3 trials, and 29 studies were in phase 3 trials.52
4.2.2 miR-485-3p antisense oligonucleotide
MicroRNAs (miRNAs) are non-coding, single-stranded RNAs that play a regulatory role in development, growth, differentiation, and neurodegenerative processes.53 miRNAs coordinate multiple signaling pathways associated with AD pathogenesis, including those involved in Aβ production and clearance, neuro-inflammation, and neurogenesis.54-56
However, little is known about the molecular mechanisms of miRNA in AD development or its applications in AD therapy. One study observed that miR-485-3p was overexpressed in the brain tissue, CSF, and plasma of patients with AD. In primary mouse neurons, miR-485-3p induced Tau hyperphosphorylation and the accumulation of cleaved Tau. Furthermore, miR-485-3p transduction was followed by a decrease in postsynaptic density protein 95 (PSD-95) levels at the synapses, an increase in the number of achaete-scute complex spots in glial cells, and the release of IL-1β,57 demonstrating miR-485-3p induces an inflammatory response in AD. In a subsequent mouse study, the injection of miR-485-3p antisense oligonucleotide (ASO) into the ventricles significantly reduced the production of insoluble Aβ 1–42 in the cortex compared with the control group. In subsequent experiments, miR-485-3p ASO enhanced Aβ phagocytosis in the microglia.58 In addition, miR-485-3p ASO was able to reduce apoptosis and Tau production.57 Although miR-485-3p ASO has shown efficacy in animal studies, it has not been applied inhuman studies. Nevertheless, miR-485-3p ASO showed promise for the treatment of AD.
4.2.3 Gamma-secretase
In the brains of patients with AD, APP was cleaved by β-secretase to produce soluble APPβ (sAPPβ) and membrane-bound APP C-terminal fragments (CTFs) (C99)59 (Figure 5). C99 was further cleaved by γ-secretase to release Aβ and the APP intracellular structural domain (AICD). Therefore, γ-secretase is a target for the treatment of AD.
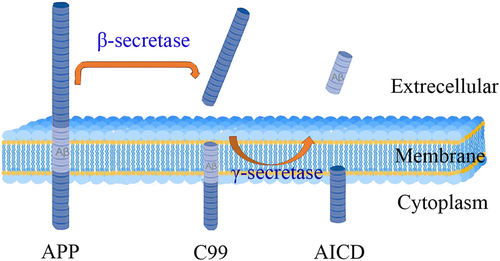
Aβ precursor protein (APP) is proteolytically hydrolyzed outside the transmembrane structural domain (TMD) by β-secretase. The remaining membrane-bound C-terminal fragment (sAPPβ) is then cleaved within the TMD to produce Aβ and APP intracellular domains (AICD).
Two types of substances act on γ-secretase: γ-secretase inhibitors (GSIs), which inhibit γ-secretase cleavage by binding to multichannel membrane proteins (PS) and reducing Aβ production,60 and γ-secretase modulators (GSMs), which modulate the γ-secretase activity that produces Aβ42.61
In a clinical setting, GSIs reduced Aβ production in patients with AD.62 However, the numerous γ-secretase substrates hindered the development of their inhibitors and caused an accumulation of APP-CTFs,63 which could lead to side effects (e.g., skin cancer, infections, and gastrointestinal bleeding). In addition, because of the bound effect of Aβ,64 the concentration of Aβ increases substantially once GSI treatment is stopped. This limits the application of these substances in clinical practice.
GSMs are superior to GSIs, and diarrhea is their most common adverse effect,65 which is well tolerated by healthy patients at unit doses. Their efficacy has been demonstrated in animal experiments; a significant reduction in Aβ deposition and microglia content was observed after long-term GSM treatment of PS/APP mice.66
4.2.4 Immunotherapy
Currently, the most refined anti-Aβ immunotherapies are vaccines and exogenous antibodies, which are classified as active and passive immunotherapy, respectively.67, 68 Thus, targeting the Aβ protein can effectively inhibit the progression of AD.
Active immunity involves the production of endogenous antibodies to the Aβ protein through the application of Aβ or its fragments.69 In a study, on a first-generation vaccine, T-cell-mediated meningoencephalitis occurred in 6% of enrolled patients with moderate to severe AD, bringing the trial to an end.70 Subsequently, a second-generation vaccine without T-lymphocyte epitopes was developed. The advantages of active immunotherapy are short-term administration, low cost, and high efficacy. However, it is difficult to predict the immune response and adverse effects.
Passive immunotherapy uses humanized monoclonal antibodies or polyclonal immunoglobulins to promote Aβ clearance.71 Unlike active immunotherapy, passive immunotherapy does not require activation of the immune response, making it safer and more predictable. However, passive immunotherapy carries the risk of edema and cerebral hemorrhage.72 Recently, several passive immunotherapy drugs for AD have entered clinical trials. For example, the FDA has approved aducanumab, a passive immune-therapy, for clinical use, although its safety is still not guaranteed. However, this Aβ antibody may slow or prevent AD-related neurodegeneration by clearing Aβ (Table 2: AD immunotherapy drug development summary42). Gammagard is a mixture of antibodies derived from human plasma that directly clears Aβ and other neurotoxic substances associated with amyloid plaques, thereby improving cognitive performance in patients. Clinical trials have shown that Gammagard can reduce amyloid plaques in the brain, but its effect on cognitive performance is unclear.73 Other promising immunotherapies include BAN2401,74 crenezumab,75 and ganteneru-mab.76
| Treatment | Drug name | Mechanism | Researchers | Role in the crowd | Management | The research phase | The results of the study | Clinical identification number | Start date | End date |
|---|---|---|---|---|---|---|---|---|---|---|
| Active immunization | AN1792 | Vaccination | Janssen/Pfizer | Patients with mild to moderate AD | IM | II | Terminated | NCT00021723 | Sep 2001 | Sep 2003 |
| Amilomotide (CAD106) | Vaccination | Novartis | Patients with mild to moderate AD | IM | II/III | Terminated | NCT02565511 | Nov 2015 | Apr 2020 | |
| UB-311 | Vaccination | United neuro-science | Mild AD | IM | II | Complete | NCT02551809 | Jan 2015 | Aug 2018 | |
| ABvac40 | Vaccination | Araclon biotech | People with amnesia mild cognitive impairment or very mild AD | SC | II | Not recruiting | NCT03461276 | Feb 2018 | Dec 2022 | |
| Passive immunotherapy | Solanezumab (LY2062430) | Monoclonal antibodies | Eli lilly | Patients with mild to moderate AD | IV | III | Complete | NCT00905372 | May 2009 | Apr 2012 |
| Patients with mild to moderate AD | Complete | NCT00904683 | May 2009 | Jun 2012 | ||||||
| Patients with mild to moderate AD | Termination of | NCT01127633 | Dec 2010 | Feb 2017 | ||||||
| Mild AD | III | Terminated | NCT01900665 | Apr 2013 | Jan 2016 | |||||
| Precursor sex AD | III | Terminated | NCT02760602 | Jun 2016 | May 2017 | |||||
| Have a memory loss risk | III | Not recruiting | NCT02008357 | Feb 2014 | Feb 2022 | |||||
| Gantenerumab | Monoclonal antibodies | Roche | Precursor sex AD | IV | Complete | NCT01224106 | Nov 2010 | Sep 2020 | ||
| Mild AD | Complete | NCT02051608 | Nov 2014 | Apr 2021 | ||||||
| Prodromal stage to mild AD | Active | NCT04374253 | Nov 2014 | Apr 2021 | ||||||
| Early AD | Active | NCT03444870 | Jun 2018 | Nov 2023 | ||||||
| Early AD | Not recruiting | NCT03443973 | Aug 2018 | Sep 2022 | ||||||
| Early AD | Not recruiting | NCT04339413 | May 2020 | Apr 2023 | ||||||
| Aducanumab | Monoclonal antibodies | Biogen | Early AD | IV | III | Terminated | NCT02484547 | Sep 2015 | Aug 2019 | |
| Early AD | III | Terminated | NCT02477800 | Aug 2015 | Aug 2019 | |||||
| Early AD | III | Not recruiting | NCT04241068 | Mar 2020 | Jan 2023 | |||||
| Crenezumab (RG7412) | Monoclonal antibodies | Roche/AC immune SA | Prodromal stage to mild AD | IV | III | Terminated | NCT02670083 | Mar 2016 | Mar 2019 | |
| Prodromal stage to mild AD | III | Terminated | NCT03114657 | Mar 2017 | Jun 2019 | |||||
| Prodromal stage to mild AD | III | Terminated | NCT03491150 | Apr 2018 | May 2019 | |||||
| Lecanemab (BAN2401) | Monoclonal antibodies | Biogen/Eisai | Early AD | IV | III | Active | NCT03887455 | Mar 2019 | Aug 2024 | |
| In preclinical AD | III | Active | NCT04468659 | Jul 2020 | Jan 2027 | |||||
| Donanemab (LY3002813) | Monoclonal antibodies | Eli lilly | Early AD | IV | III | Active | NCT04437511 | Jun 2020 | Dec 2023 | |
| In preclinical AD | III | Active | NCT05026866 | Aug 2021 | Sep 2027 |
Although some drug research has failed to yield the desired results, this research has laid the groundwork for further studies. Viscerotropic hemostatic agents, which are vasoactive drugs, increase platelet aggregation and coagulation, thus promoting the hemostatic response. However, these drugs may aggravate pre-existing vascular lesions and circulation disorders, which are associated with AD pathogenesis.77
Solanezumab is an antibody drug that targets the clearance of Aβ. Because patients with AD have excess Aβ deposits in their brains, solanezumab may alleviate the disease. However, multiple clinical trials have shown no significant effects of solanezumab on AD outcomes. The results of the most recent phase 3 trial, which included 2100 participants worldwide, were published in 2016, but the primary clinical endpoint was not met. However, several secondary clinical endpoints were met; the most significant results were observed in patients with early and mild AD. Although some evidence suggests that solanezumab may slow the progression of cognitive impairment, it does not completely cure or reverse the development of AD.78
5 NON-PHARMACOLOGICAL TREATMENT
Current drug treatments relieve AD symptoms, but do not address the cause of the disease, and it is difficult to unify drug administration methods. Four drug delivery methods are available: oral administration, intravenous injection, intracerebral administration, and intranasal administration. Because of the intestinal barrier and BBB, it is difficult for drugs administered orally and intravenously to reach the targetlocation.79 Direct intracerebral administration is expensive and causes high levels of pain, which greatly reduces patient compliance. In contrast, intranasal administration can bypass the BBB; it is noninvasive, painless, and simple, and it can be administered without a healthcare professional.80, 81 However, the intranasal volume is small, so highly concentrated drugs must be used. In addition, the mechanism by which intranasal drug delivery bypasses the BBB is unclear, and the drug effect and route of transport are unknown. These factors limit the development of this technology. Therefore, the search for drugs and therapies that act directly on the patient's lesion location has become a popular research direction.
5.1 Music therapy
Music therapy (musicotherapy) is emerging as an alternative interdisciplinary technique that integrates music, medicine, and psychology. In 2012, Cuddy et al. found that music memory is separate from temporal lobe memory,82 and that musical memory is not lost until late in the disease.83 Moreover, musical memory is not monolithic; whenever musical memory is activated, it simultaneously activates broad memory networks in the brain. Behavioral studies have shown that music improves autobiographical recall,84 and musical recall shares the same principles as involuntary recall. Therefore, involuntary recall can be triggered during musical recall, and these memories are specific and capable of generating emotional responses. A systematic review of 38 non-drug interventions found that music therapy was the most effective therapy, especially for agitation and anxiety.85, 86 Studies by Jun LV and Jun Zhang have shown that music therapy can significantly improve the mental states of patients with AD and their caregivers, for example, by reducing anxiety.87
There are still some challenges involved in using music therapy for the treatment of AD. One of the main challenges is to ensure the quality and effectiveness of music therapy. Music therapy must be carefully designed and implemented to ensure that its effects meet clinical needs, and can be replicated in differentpatients.88
5.2 Intestinal flora
Recent studies have shown that the connection between the gut and the brain, or the gut-brain axis, is bidirectional.89 Cattaneo et al. observed pro-inflammatory bacteria in the gut of patients with AD.90 In addition, several studies have shown that alterations in the gut flora directly contribute to cognitive decline and are involved in the progression of AD.91-93 Dietary habits directly affect the composition of the gut flora; for example, the Mediterranean diet (a diet rich in vegetables, fruits, whole grains, nuts, and olive oil, with moderate consumption of fish and poultry and limited consumption of red meat and sweets) may delay neurodegeneration, and green vegetables and berries may be more effective in cognitive decline compared with other vegetables and fruits.94 A study by Varesi et al. reported that patients who received probiotics (live microorganisms with low or zero pathogenicity that provide beneficial health effects95) had significantly improved MMSE scores after 12 weeks compared with controls (p < 0.001)96; furthermore, improvements in plasma malon-dialdehyde, serum C-reactive protein, β-cell function, serum triglycerides, and quantitative control indices of insulin sensitivity were observed in individuals receiving a probiotic mixture.97 Similarly, a meta-analysis found that patients treated with probiotics exhibited significantly improved cognition and sustained reductions in malondialdehyde and high-sensitivity C-reactive protein levels after the intervention compared with control groups.98
One study used fecal flora transplantation (FMT) to transplant the fecal flora from AD mice into the gut of normal mice and detected cognitive impairment, reduced brain-derived neurotrophic factor expression, increased memory impairment, increased levels of circulating pro-inflammatory cytokines, and Aβ plaque deposition in the normal mice.99, 100 To date, the scientific community has not identified a definite flora composition that is associated with AD; however, research has established a relationship between intestinal flora and AD and determined that specific dietary habits may prevent and alleviate AD. Therefore, intestinal flora-based interventions could represent a simple and useful approach to treating AD.
5.3 Acupuncture
Traditional Chinese Medicine (TCM) is an ancient traditional medical system developed in China that has been used for thousands of years. It is a comprehensive science focusing on the transformation of health and disease, including disease prevention, diagnosis, treatment, and rehabilitation based on TCM theory and practical experience. TCM theory is rooted in medical experience and the principles conceived in ancient China, including the theory of essence, Yin and Yang and the five elements, Qi and the blood and body fluid, Visceral manifestation, channels and collaterals, constitution, etiology, pathogenesis, pathogenesis, treatment, and health preservation.101
In China, acupuncture has long been used to treat neurological disorders, including dementia, Parkinson's disease, stroke, and sleep disorders.102 Meta-analysis suggested significant superiority of acupuncture over medication therapy with regard to efficacy rate, MMSE scores, ADL scores, and ADAS-cog scores by Huang Qi and Luo Dan, who found that acupuncture was more effective than drug treatment.103 The efficacy of acupuncture was evaluated using clinical efficacy rates, the MMSE, the Ability to Perform Daily Living (ADL) scale, the AD Assessment Scale-Cognitive Score, and the Hasegawa Dementia Scale (HDS), and acupuncture led to improvements (95% CI: −0.26–0.90, Z = 0.35, p = 0.73) on all scales except the HDS, and the rate of adverse events in the drug-only control group was 13%.
Acupuncture therapy integrates the acupuncture meridians of TCM with modern medical treatments. Acupuncture targeting GV20 and GV14 (governor vessel points), EX-HN1 (a value point), KI1 and KI3 (kidney meridian points), LR3 (a liver meridian point), BL23 (a bladder meridian point), ST36 and ST40 (stomach meridian points), SP10 (a spleen meridian point), and RN4 and RN6 (Ren meridian points) down-regulated the concentrations of Aβ1-40 and Aβ1-42 in humans.104 Furthermore, acupuncture targeting GV20, GV14, and BL23 reduced Tau protein expression and enhanced learning memory in mice.105 Clinically proven acupuncture targeting GV20, GV26, and EX-HN3 downregulated the expression of NLRP3 and other inflammatory factors (e.g., IL-1β),106 ameliorating neuroinflammation.
Research has demonstrated that acupuncture can reduce Aβ deposition in the hippocampus and related brain areas to alleviate AD-induced pathological changes, but it may have other physiological effects in patients with AD, such as increasing the release of cholinergic neurotransmitters, improving cognitive function and memory, regulating the immune system and the inflammatory response, and inhibiting neuronal damage and degenerative changes. Notably, the use of acupuncture for AD has been recognized by the NIH and is included in the US health insurance system.104
5.4 Stem cell therapy
Drugs cannot address the neuronal death, synaptic deficits, and brain atrophy that characterize AD pathology, and the differentiation potential of stem cells could solve this dilemma.107 Commonly used stem cell types are brain-derived neural stem cells (NSCs), bone marrow-derived mesenchymal stem cells (BM-MSCs), human umbilical cord blood-derived mesenchymal stem cells (HUCB-MSCs), and embryonic stem cells (ESCs).108
Transplanted NSCs can compensate for neuron loss and directly repair tissue damage. Moreover, consistent NSCs can produce paracrine cytokines that have indirect neural effects. NSC transplantation can improve cognitive impairment, memory, and learning deficits in rodents.109 NSCs can also have adverse effects because they differentiate into non-neuronal glial cells.110 Non-neuronal glial cells can secrete excessive inflammatory factors and activate neurons and immune cells, thus leading to neuronal death and the exacerbation of the inflammatory response, further promoting the development of neurodegenerative diseases.111
One of the main modes of action of BM-MSCs is inflammation reduction. Inflammation is known to play a crucial role in the pathology of AD, contributing to the accumulation of amyloid plaques, the loss of synapses, and neuronal degeneration. BM-MSCs produce a variety of anti-inflammatory cytokines and growth factors, including interleukin-10 (IL-10), transforming growth factor β (TGF-β), and hepato- cyte growth factor (HGF), which inhibit brain microglia activation and reduce inflammation. In addition, BM-MSCs can also reduce the oxidative stress in AD. Oxidative stress is a process that produces reactive oxygen species and contributes to the destruction of neurons and other brain cells.112 BM-MSCs can produce anti-oxidants, such as glutathione, superoxide dismutase (SOD), and catalase, which scavenge free radicals and reduce oxidative stress in the brain. Finally, BM-MSCs promote neurogenesis in the hippocampus, a critical region for learning and memory that is heavily affected by AD. BM-MSCs can differentiate into nerve cells and support the growth and survival of existing neurons, thereby improving cognitive function and memory.113 However, BM-MSCs can have adverse effects due to multi- organ infiltration when injected intravenously, and transplanted BM-MSCs may cause thrombosis during treatment.114
Compared with other stem cells, HUCB-MSCs are noninvasive and exhibit low immunogenicity and superior wizardry.115 A previous study showed that HUCB- MSCs can release various cytokines, such as neurotrophic factors, hormones, and neurotransmitters, thereby reducing the AD-related inflammatory response caused by neuronal injury and inhibiting microglial activation.116 In addition, HUCB-MSCs can reduce Aβ42-induced synaptic defects by promoting platelet adhesion through thrombospondin-1 (TSP-1) secretion, thus providing an effective treatment strategy for early AD.117
ESC-based treatment for AD is currently in the laboratory stage. The premise of this treatment is to cultivate ESCs into neurons or other types of brain cells, and transplant these cells into the brains of patients with AD to improve their neural function and cognitive ability. ESC transplantation has shown potential efficacy in animal experiments, but no clinical trials have been carried out yet, and several safety and ethical issues remain. These include ethical issues related to the source of ESCs, the possibility of rejection reactions during the transplantation process, and the possibility of the abnormal proliferation of ESCs, leading to adverse reactions (e.g., tumor formation). Therefore, although ESC technology may provide new ideas and solutions for AD treatment in the future, additional research on the safety and scope of ESCs is still needed. Other treatment methods that are more established and feasible, such as biological agents, gene therapy, drug therapy, and non-drug therapy, also need to be explored further.118
Although stem cell therapy for AD is still in the laboratory and clinical research stages, the development of this treatment has attracted widespread attention, and many scientists believe that stem cell therapy is a vital research direction. In the coming years, technological advancements and additional research will enable increased development and application of this treatment. The current research on stem cell therapy is summarized in Table 3.108
| Stem cell types | Advantages | Limitations |
|---|---|---|
| NSCs | Multipotent; easy adaption in brain; no need for transdifferentiating | Invasive collection; poor survival; tumorigenesis; non-neuronal glia; intrahippocampal or intraventricular stereotactic injection |
| BM-MSCs | Autologous transplantation; easy handling; multipotent; intravenous application; phase-I/II clinical trials | Low rate of neuronal differentiation; tumorigenesis; thrombosis; poor homing and multiple organ infiltration |
| hUCB- MSC | Noninvasive collection; easy handling; multipotent; phase-I/II a clinical trial | Ethical and immunogenic issues; tumorigenesis; poor homing; stereotactic brain injection |
| ESCs | Unlimited self-renewal; pluripotent | Ethical and immunogenic issues; uncontrolled differentiation and teratoma formation; only a few studies in experimental animals |
| iPSCs | Multipotent; autologous; multipotent | Only a few studies in experimental animals; possible pathological phenotype |
5.5 Nanoparticle technology treatment
Nanoparticles are nanomaterials that carry drugs by adsorption, encapsulation, chemical bonding, and molecular combination. Nanotechnology has provided treatment strategies for any human disorder, including AD. The current clinical trials on nanoparticles for the treatment of AD are mainly focused on drug delivery and brain imaging. Although drugs that can treat AD are available, the onset region of AD is intracranial, and the majority of macromolecular drugs and 98% of micromolecular drugs cannot reach the target site because of the BBB.46 By contrast, nanoparticles can be developed in different sizes, and researchers can select the physical, chemical, and optical properties to improve the stability, bioavailability, and targeting of nanoparticles.119 Many studies have shown that nanoparticles can be successfully modified with bioactive compounds with strong antioxidant and anti-inflammatory effects; these nanoparticles can enter the target brain sites to prevent and control neurological diseases120 (Figure 6).
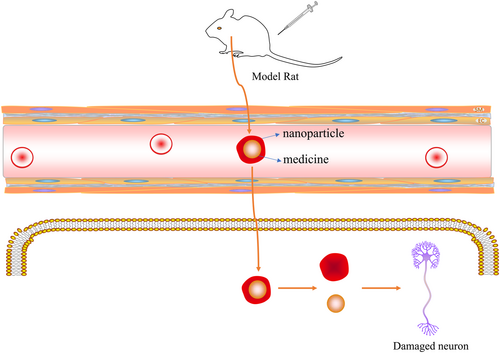
The nanoparticles carry drugs across the blood-brain barrier (BBB) to target cells.
Not only can nanoparticles be used to carry drug compounds, but those made with metals can also have direct therapeutic effects in patients. Chernyet al. showed that copper and zinc chelating agents reversed Aβ deposition in patients with AD, thus improving their cognitive performance.121 However, when treating AD with nanoparticle technology, it is still necessary to control the dose and monitor safety. Nanoparticles that are too small may cross the BBB, leading to adverse effects on neurons. Therefore, further research and development of nanoparticle technology is needed to optimize its dosage, safety, and ultimate clinical effect.46
In general, nanoparticle technology provides a variety of therapeutic strategies. Future research is necessary to elucidate its mechanism of action and safety and explore its potential application in the treatment of AD.
6 DISCUSSION
AD is considered a major global health problem. In 2011, the Alzheimer's Association of America established more reliable diagnostic criteria for AD, designating Aβ and neuronal damage as markers for AD to improve the specificity and sensitivity of diagnosis. However, AD has no cure; the three currently approved drugs are only able to alleviate symptoms. Therefore, scientists continue to search for a true cure for AD. Studies have shown that improving a patient's lifestyle and diet can improve brain health and reduce the risk of AD. For example, music therapy and the Mediterranean diet, a non-pharmacological intervention that is currently the first line of treatment. Acupuncture has a significant role in the treatment of AD, and when evaluated by a scale, acupuncture therapy significantly improves symptoms; and the incidence of adverse events is much lower than that with Western medical treatment. Thus, TCM has a great potential in the treatment of AD. Improvements in science and technology have enabled stem cell and nanoparticle research to advance; these highly sophisticated technologies are promising, although they are not yet used in clinical settings. However, animal experiments have shown promising results.
Despite the strong focus on AD research in recent years, new therapies and technologies have many shortcomings. The available therapies for AD are only applicable in early and mid-stage cases; they have minimal effects on the symptoms of patients with advanced disease. This indicates that neuronal damage and cognitive dysfunction in advanced stages are difficult to reverse. The failure rate of clinical trials for AD treatments has been reported to be as high as 99.6%.122 For example, the high price of sophisticated technological therapies, such as stem cell and nanoparticle technology therapies, greatly limits their clinical application. Therefore, we reviewed the therapies that have been applied and those that are currently being investigated and have shown promise for clinical use, including pharmacotherapy, music therapy, and non-pharmacological treatments. This review demonstrates that further research is needed to determine the effectiveness of these treatments. We hope that future studies will contribute to the discovery of new therapeutic approaches to cure AD and that scholars will continue to make breakthroughs to develop new, effective therapies that will extend patients' healthy lifespans and reduce the burden on the patients, their caregivers, and society.
7 STRENGTHS AND LIMITATIONS
In this review, our team consulted the literature to select and describe the most cutting-edge and promising AD treatment methods, thereby providing ideas for subsequent research.
However, this review has several limitations. First, our literature review was limited, and other treatment methods may exist. Therefore, we will continue to study AD. Second, the non-drug therapies described in this paper are new, and few reports are available in the literature. In a follow-up study, our team will study one of these methods to contribute to the treatment of patients with AD.
AUTHOR CONTRIBUTIONS
Can Mei: Investigation, resources, software, writing – original draft. Jianbo Zhan: Investigation, resources. Shuzhen Zhu: Resources, supervision. Yutong Zhang: Investigation, resources, supervision. Chang-e Xiong: Investigation, resources, software, supervision. Jia Wang: Investigation, resources, software, supervision. Yu Jia Xu: Investigation, resources, supervision. Hua Zhong: Supervision, validation, writing – review & editing. Jing Cheng: Investigation, project administration, resources, software, supervision, validation, writing – review & editing.
ACKNOWLEDGMENTS
This work was supported by the Science and Technology Department of Hubei Province (2022CFB015), the Hubei Province Key Laboratory of Occupational Hazard Identification and Control (OHIC2019G04), the Education Department of Hubei Province (19Q016), and Wuhan University of Science and Technology (2019x076). We also thank the School of Public Health, Wuhan University of Science and Technology, Hubei Province Center for Disease Control and Prevention, in particular Mr. Jian bo Zhan, Shu zhen Zhu, and a counselor, who assisted in the study design. We also thank the Chinese Center for Disease Control and Prevention and Ms. Yu tong Zhang for participating in discussions of the final draft. The authors acknowledge Jing Cheng for her valuable ideas.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicting interests.
ETHICS STATEMENT
Ethics approval was not needed for this study.
Abbreviations
-
- AD
-
- Alzheimer’s disease
-
- ADL
-
- Ability to Perform Daily Living Scale
-
- AICD
-
- APP intracellular structural domain
-
- APP
-
- Aβ precursor protein
-
- AUC
-
- area under the curve
-
- Aβ
-
- β-amyloid
-
- BBB
-
- blood-brain barrier
-
- BM-MSCs
-
- bone marrow-derived mesenchymal stem cells
-
- CSF
-
- cerebrospinal fluid
-
- ESCs
-
- embryonic stem cells
-
- FMT
-
- fecal flora transplantation
-
- HAMA
-
- Hamilton Anxiety Inventory
-
- HAMD
-
- Hamilton depression scale
-
- HDS
-
- Hasegawa Dementia Scale
-
- HGF
-
- hepatocyte growth factor
-
- HUCB-MSCs
-
- human umbilical cord blood-derived mesenchymal stem cells
-
- IL-10
-
- interleukin-10
-
- IWG
-
- International Alzheimer's Disease Task Force
-
- MCI
-
- moderate cognitive impairment
-
- miRNAs
-
- MicroRNAs
-
- MMSE
-
- Mini-Mental State Examination
-
- MRI
-
- magnetic resonance imaging
-
- MTDs
-
- multi-targeted drugs
-
- NFTs
-
- neurofibrillary tangles
-
- NIA-AA
-
- National Institute on Aging - Alzheimer's Disease Association Working Group
-
- NINCDS-ADRDA
-
- National Institute on Aging - Alzheimer's Disease Association
-
- NMDA
-
- N-methyl d-aspartate
-
- NSCs
-
- neural stem cells
-
- PSQI
-
- Pittsburgh Sleep Quality Index
-
- SES
-
- Self-Esteem Scale
-
- SOD
-
- superoxide dismutase
-
- SRHMS
-
- Self-Rated Health Scale
-
- SWLS
-
- Satisfaction with Life Scale
-
- TCM
-
- Traditional Chinese medicine
-
- TGF-β
-
- transforming growth factor-β
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.




