Cardiac troponin and cerebral herniation in acute intracerebral hemorrhage
Abstract
Objectives
To explore the association, if any, between the relationship between cardiac troponin and cerebral herniation after intracerebral hemorrhage (ICH).
Methods
Six hundred and eighty-seven consecutive ICH patients admitted to West China Hospital from May 1, 2014 to September 1, 2015 were retrospectively reviewed. Data on demographics, etiology, laboratory examinations at admission including serum cardiac troponin, computed tomography (CT) scans at admission and follow-up, and clinical outcomes were obtained. Using multiple logistic regression to identify the relationship of troponin and herniation. The association between troponin and hematoma volume was assessed using bivariate correlation and linear regression.
Results
Among 188 (27.4%) patients who underwent the test of serum cardiac troponin at admission, 16 (8.5%) demonstrated cerebral herniation. The median time from symptom onset to CT at admission and follow-up was 4 and 30.25 hr, respectively. In multivariate analysis, elevated troponin was independently associated with cerebral herniation (adjusted odds ratio [OR] 5.19; 95% confidence interval [CI], 1.08–24.93). And those with elevated troponin had larger hematoma volume at follow-up in bivariate correlation (correlation coefficient, .375, p = .003) and linear regression (β, .370, 95% CI, 0.062–0.320, p = .005), higher National Institutes of Health Stroke Scale score (adjusted OR 2.06; 95% CI, 1.06–4.01, p = .033) and lower Glasgow Coma Scale score (adjusted OR 2.34; 95% CI, 1.17–4.68, p = .016) than those without.
Conclusions
Elevated cardiac troponin was associated with an almost five-fold increased risk of cerebral herniation, but not in-hospital mortality. The possibility of cerebral herniation should be considered when ICH patients with large hematoma volume and elevated troponin.
1 Introduction
Cerebral herniation is a severe complication after intracerebral hemorrhage (ICH) which often results in the dysfunction of brain stem and cranial nerves (Gower, Baker, Bell, & Ball, 1987; Koenig et al., 2008) and requires immediate diagnosis and neurological life support (Stevens, Shoykhet, & Cadena, 2015). The recognition of cerebral herniation is very important due to its high mortality.
On the other hand, serum cardiac troponin is closely associated with vascular events (Everett, Zeller, Glynn, Ridker, & Blankenberg, 2015) and has been widely used as the biomarker for myocardial infarction and cardiac damage after stroke (Naidech et al., 2005). Recently, elevated troponin has been found to be associated with poor outcome and mortality in stroke patients (Batal et al., 2016; Raza & Alkhouli, 2014; Thalin et al., 2015). Thus, serum troponin levels might play a pivotal role in stroke patients and are associated with the severity of stroke. However, most of the studies on cardiac troponin and stroke were performed in patients with cerebral infarct or subarachnoid hemorrhage (SAH; Batal et al., 2016; Zhang, Wang, & Qi, 2015). Little is known about the effect of cardiac troponin in ICH patients, especially the relationship of elevated troponin and cerebral herniation, as well as the association between elevated troponin and hematoma volume.
Thus, we aimed to explore the association, if any, between serum troponin and cerebral herniation, as well as hematoma volume after ICH.
2 Methods
2.1 Patients
Within the Scientific Research Department of West China Hospital approval, we retrospectively reviewed ICH patients who had the test of serum cardiac troponin at admission. We identified 687 consecutive computed tomography (CT)-positive ICH patients admitted to West China Hospital from May 1, 2014 to September 1, 2015. Traumatic ICH, primary SAH and Hemorrhagic transformation of cerebral infarct were excluded. Among the 687 ICH patients, 188 (27.4%) had the test of serum cardiac troponin at admission. The methodology of our study was in accordance with local ethics criteria for human research. All patients or their guardians provided informed consent for participation.
2.2 Data collection
We derived data on demographics, doubtful risk factors for ICH, data of electrocardiogram, clinical characteristics and time to CT from the prospective database of the National Key Technology R&D Programme of the 12th Five-Year Plan which had been reported previously (Lei et al., 2015). The severity on admission was measured using the Glasgow Coma Scale (GCS) and the National Institutes of Health Stroke Scale (NIHSS; Brott et al., 1989) by certified physicians.
The biomarkers related to coagulation function, renal function, cardiac biomarkers including cardiac troponin, myoglobin, and creatine kinase-MB mass (CK-MB), and serum ions. Blood samples were taken and processed as soon as possible while at admission. Imaging information (cerebral herniation, hematoma volume, the location of hematoma, presence of intraventricular hemorrhage, combined with SAH) is limited to CT scans or CTA. Etiology of ICH was categorized as hypertension, aneurysm, arteriovenous malformation, moyamoya disease, severe systemic disease, or unknown. Data on in-hospital mortality was also collected. Available CT scans were independently reviewed by M.X. and J.L.
2.3 Definitions
On CT scans, cerebral herniation was characterized by uncontrollable diffuse brain swelling or mass effect resulted from predominantly unilateral brain swelling, often combined with obliteration of perimesencephalic cisterns (Bor-Seng-Shu et al., 2013). Hematoma volume was calculated using the formula of ABC/2 where A is the maximum diameter of the greatest cross section of the hematoma, B is perpendicular to A and C represents the slice thickness multiplied by the number of CT slices (Kothari et al., 1996). The volume of hemorrhage flooding into ventricular was not included into the measurement of hematoma volume. The location of hematoma was categorized into deep, lobe, posterior fossa, primary intraventricular hemorrhage and multilple/undefined. Large hematoma volume was defined as over 30 ml. Elevated troponin was defined as over 14 ng/L. Elevated myoglobin was defined as over 58 ng/ml. Elevated CK-MB was defined as over 4.94 ng/ml. Using the Formula of Modification of Diet in renal disease to calculate estimated glomerular filtration rate (eGFR): eGFR = 186 × (Scr)−1.154 × (age)−0.203 × (0.742 if female; National Kidney Foundation, 2002). Chronic kidney disease (CKD) was defined as eGFR < 60 ml/min per 1.73 m2. End stage renal disease (ESRD) was defined as eGFR < 15 ml/min per 1.73 m2.
2.4 Statistics
Baseline characteristics were compared using the χ2 test or fisher exact test for categorical data, and Student t test or Mann–Whitney U test for continuous data, as appropriate. Using multivariate logistic regression to study the association between elevated serum troponin and the incidence of cerebral herniation. The association between troponin and hematoma volume was assessed using bivariate correlation and linear regression. In the linear regression, we adjusted for age, GCS score and systolic blood pressure at admission.
3 Results
During the study period, a total of 188 (27.4%) patients were enrolled. Among all these 188 subjects with complete data, mean age was 56.34 years. 60.6% were men, and 66 (35.1%) had elevated serum cardiac troponin. The median time from symptom onset to CT at admission and follow-up was 4 and 30.25 hr, respectively. The mean hematoma volume was 30.04 ml (median, 24.34, range, 32.05) and 39.9% were originated in deep.
Among these 66 patients with elevated troponin, one had cardiac ischemia characterized by atrial premature beats, the change of T-wave and left anterior fascicular block on electrocardiogram; other electrocardiogram abnormalities included atrial fibrillation in one patient and supraventricular tachycardia in one patient. In addition, 5 of the 66 patients had pre-existing heart disease which included coronary heart disease, myocardial infarction and congenital heart disease.
Table 1 showed the baseline clinical characteristics between patients with and without cerebral herniation. Most of the characteristics were comparable, but subjects with cerebral herniation were more likely to have lower GCS score (p = .003), higher NIHSS score (p = .003), higher proportion of elevated serum troponin (p = .016) and larger hematoma volume (p = .002) compared with patients without cerebral herniation.
| Characteristic | Total | Non-cerebral hernia (n = 172) | Cerebral hernia (n = 16) | p Value |
|---|---|---|---|---|
| Age, mean (SD) | 56.34 (16.79) | 56.31 (16.85) | 56.56 (16.67) | .955 |
| Male, % | 114 (60.6) | 101 (58.7) | 13 (81.3) | .078 |
| Hypertension, % | 87 (46.3) | 78 (45.3) | 9 (56.3) | .403 |
| Diabetes mellitus, % | 16 (8.5) | 15 (8.7) | 1 (6.3) | 1.000 |
| Cardiac events, % | 14 (7.4) | 13 (7.6) | 1 (6.3) | 1.000 |
| Previous stroke, % | 21 (11.2) | 20 (11.6) | 1 (6.3) | .812 |
| Alcohol intake, % | 29 (15.4) | 28 (16.3) | 1 (6.3) | .484 |
| Smoking, % | 49 (26.1) | 45 (26.2) | 4 (25) | 1.000 |
| Systolic blood pressure, mean (SD) | 160.29 (34.02) | 159.16 (34.21) | 172.25 (30.36) | .142 |
| Diastolic blood pressure, mean (SD) | 94.68 (20.93) | 94.24 (20.93) | 99.25 (21.05) | .362 |
| GCS, median (IQR) | 12 (9) | 12 (8) | 6 (7.5) | .003 |
| NIHSS, median (IQR) | 10 (18.5) | 10 (15.75) | 27.5 (19.25) | .003 |
| Coagulation function | ||||
| PT, mean (SD) | 13.07 (9.17) | 13.17 (9.55) | 11.99 (1.06) | .658 |
| APTT, mean (SD) | 27.73 (6.84) | 27.81 (7.02) | 26.80 (4.31) | .611 |
| INR, mean (SD) | 1.11 (0.43) | 1.11 (0.45) | 1.07 (0.09) | .763 |
| Fibrinogen, mean (SD) | 3.07 (0.97) | 3.09 (0.97) | 2.88 (0.98) | .463 |
| Renal function | ||||
| Urea nitrogen, mean (SD) | 6.45 (3.98) | 6.46 (4.06) | 6.39 (2.83) | .951 |
| eGFR, mean (SD) | 93.55 (34.04) | 93.64 (34.29) | 92.53 (32.32) | .901 |
| CKD, % | 24 (12.8) | 22 (12.8) | 2 (12.5) | 1.000 |
| ESRD, % | 6 (3.2) | 6 (3.5) | 0 | 1.000 |
| Cardiac biomarkers | ||||
| Elevated troponint, % | 66 (35.1) | 56 (32.6) | 10 (62.5) | .016 |
| Elevated myoglobin, % | 123 (65.4) | 114 (66.3) | 9 (56.3) | .420 |
| Elevated CK-MB, % | 6 (3.2) | 6 (3.5) | 0 | 1.000 |
| Serum ions | ||||
| Sodium, mean (SD) | 138.35 (5.99) | 138.32 (6.08) | 139.15 (3.88) | .851 |
| Potassium, mean (SD) | 3.53 (0.47) | 3.54 (0.48) | 3.35 (0.63) | .584 |
| Phosphorous, mean (SD) | 0.91 (0.43) | 0.91 (0.44) | 0.89 (0.01) | .949 |
| Calcium, mean (SD) | 2.19 (0.32) | 2.19 (0.33) | 2.19 (0.98) | .979 |
| Magnesium, mean (SD) | 0.83 (0.16) | 0.83 (0.17) | 0.79 (0.07) | .734 |
| Hematoma volume, mean (SD) | 30.04 (26.39) | 26.31 (22.15) | 64.18 (36.95) | .002 |
| Change in hematoma volume from diagnostic to follow up CT, mean (SD) | 2.20 (11.42) | 5.56 (18.60) | 1.95 (10.86) | .451 |
| Hematoma site | ||||
| Lobe, % | 64 (34) | 60 (34.9) | 4 (25) | .425 |
| Deep, % | 75 (39.9) | 69 (40.1) | 6 (37.5) | .838 |
| Posterior fossa, % | 26 (13.8) | 24 (14) | 2 (12.5) | 1.000 |
| Primary intraventricular hemorrhage, % | 11 (5.9) | 10 (5.8) | 1 (6.3) | 1.000 |
| Multiple/undefined, % | 12 (6.4) | 9 (5.2) | 3 (18.8) | .114 |
| Intraventricular hemorrhage, % | 84 (44.7) | 76 (44.2) | 8 (50) | .655 |
| Combined with subarachnoid hemorrhage | 27 (14.4) | 26 (15.1) | 1 (6.3) | .552 |
| Model 1 OR (95% confidence interval)a | 1 | 3.28 (1.08–9.96) | .037 | |
| Model 2 OR (95% confidence interval)b | 1 | 5.19 (1.08–24.93) | .040 | |
- SD, standard deviation; IQR, interquartile range; GCS, the Glasgow Coma Scale; NIHSS, the National Institutes of Health Stroke Scale; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international normalized ratio; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; ESRD, end stage renal disease; CK-MB, creatine kinase-MB mass.
- a Adjusted for age, sex, GCS and NIHSS.
- b Adjusted for additional history of hypertension, diabetes mellitus, cardiac events, previous stroke, smoking, systolic blood pressure on admission, chronic kidney disease and hematoma volume >30 ml.
After adjusting for age, sex, GCS and NIHSS, those with cerebral herniation were more likely to have elevated troponin (odds ratio [OR] 3.28, 95% confidence interval [CI], 1.08–9.96, p = .037). After adjusting for additional confounders, elevated serum troponin was still associated with the presence of cerebral herniation (OR, 5.19; 95% CI, 1.08–24.93, p = .040).
Next, the association between elevated troponin and the location of hematoma, as well as the etiology and the severity of ICH, was analyzed (Table 2). Patients with elevated troponin were older (p = .003), more likely to have diabetes mellitus (p = .016), had higher systolic blood pressure and diastolic blood pressure (p = .004, p = .013, respectively). As for the hematoma site, patients with elevated troponin had a higher proportion of hematoma in deep compared with those with normal troponin (50% vs. 34.4%, p = .037), and were more likely to be accompanied by hypertensive ICH (p < .001), whereas normal troponin was more likely to be accompanied by secondary ICH such as arteriovenous malformation (p = .034). In addition, patients with elevated troponin had lower GCS score and higher NIHSS score (p = .026, .005, respectively). And in-hospital mortality was also higher among elevated troponin group compared with normal troponin group (16.7% vs. 4.9%, p = .007). After adjusting for only age and sex, elevated troponin was significantly associated with GCS < 8, as well as NIHSS > 10, whereas the relationship of elevated troponin with in-hospital mortality and deep location of ICH became no longer significant (Table 3). After adjusting for additional confounders such as history of hypertension, diabetes mellitus, smoking, previous stroke and systolic blood pressure, elevated troponin was still associated with GCS < 8 (adjusted OR 2.34; 95% CI, 1.17–4.68, p = .016) and NIHSS > 10 (adjusted OR 2.06; 95% CI, 1.06–4.01, p = .033).
| Elevated troponin (n = 66) | Normal troponin (n = 122) | p Value | |
|---|---|---|---|
| Age, mean (SD) | 61.17 (17.12) | 53.72 (16.08) | .003 |
| Male, % | 45 (68.2) | 69 (56.6) | .119 |
| Hypertension, % | 36 (54.5) | 51 (41.8) | .094 |
| Diabetes mellitus, % | 10 (15.2) | 6 (4.9) | .016 |
| Cardiac events, % | 5 (7.6) | 9 (7.4) | 1.000 |
| Previous stroke, % | 8 (12.1) | 13 (10.7) | .761 |
| Alcohol intake, % | 7 (10.6) | 22 (18) | .178 |
| Smoking, % | 17 (25.8) | 32 (26.2) | .944 |
| Systolic blood pressure, mean (SD) | 170 (36.76) | 155.03 (31.36) | .004 |
| Diastolic blood pressure, mean (SD) | 100.26 (23.88) | 91.65 (18.56) | .013 |
| CKD | 20 (30.3) | 4 (3.3) | <.001 |
| ESRD | 6 (9.1) | 0 | .003 |
| Hematoma volume at admission, mean (SD) | 35.10 (29.41) | 26.85 (23.91) | .084 |
| Hematoma site | |||
| Lobe, % | 13 (19.7) | 51 (41.8) | .002 |
| Deep, % | 33 (50) | 42 (34.4) | .037 |
| Posterior fossa, % | 8 (12.1) | 18 (14.8) | .618 |
| Primary intraventricular hemorrhage, % | 6 (9.1) | 5 (4.1) | .286 |
| Multiple/undefined, % | 6 (9.1) | 6 (4.9) | .421 |
| IVH, % | 36 (54.5) | 48 (39.3) | .045 |
| Etiology | .005 | ||
| Hypertension, % | 53 (80.3) | 66 (54.1) | <.001 |
| Aneurysm, % | 5 (7.6) | 18 (14.8) | .152 |
| Arteriovenous malformation, % | 2 (3.0) | 15 (12.3) | .034 |
| Moyamoya disease, % | 3 (4.5) | 1 (0.8) | .246 |
| Severe systemic disease | 0 | 5 (4.1) | .164 |
| Unknown | 6 (9.1) | 19 (15.6) | .211 |
| Severity of stroke | |||
| GCS, median (IQR) | 10 (7.5) | 13 (8.25) | .026 |
| NIHSS, median (IQR) | 12 (20) | 8.5 (16.25) | .005 |
| In-hospital mortality, % | 11 (16.7) | 6 (4.9) | .007 |
- SD, standard deviation; IQR, interquartile range; GCS, the Glasgow Coma Scale; NIHSS, the National Institutes of Health Stroke Scale; CKD, chronic kidney disease; ESRD, end stage renal disease; IVH, intraventricular hemorrhage.
| Outcome | Category | Adjusted OR (1) | Adjusted 95% CI (1) | p Value | Adjusted OR (2) | Adjusted 95% CI (2) | p Value |
|---|---|---|---|---|---|---|---|
| GCS<8 | Elevated troponin | 2.60 | 1.34–5.05 | .005 | 2.34 | 1.17–4.68 | .016 |
| Normal troponin | Reference | — | Reference | — | |||
| NIHSS > 10 | Elevated troponin | 2.34 | 1.24–4.41 | .008 | 2.06 | 1.06–4.01 | .033 |
| Normal troponin | Reference | — | Reference | — | |||
| In-hospital mortality | Elevated troponin | 2.63 | 0.87–7.93 | .085 | 2.24 | 0.69–7.28 | .179 |
| Normal troponin | Reference | — | Reference | — | |||
| Deep location of ICH | Elevated troponin | 1.64 | 0.87–3.08 | .126 | 1.32 | 0.67–2.61 | .424 |
| Normal troponin | Reference | — | Reference | — |
- Adjusted (1): adjusted for age and sex. Adjusted (2): adjusted for age, sex, history of hypertension, diabetes mellitus, smoking, previous stroke and systolic blood pressure.
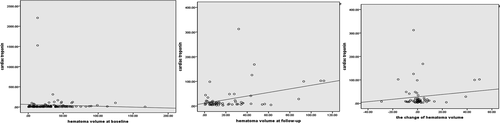
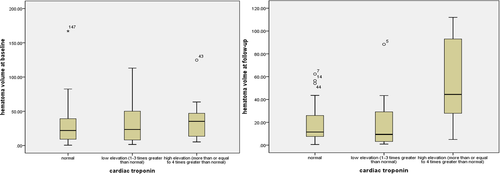
As for hematoma volume, those with elevated troponin had large hematoma volume at follow-up in bivariate correlation (correlation coefficient, .375, p = .003) and linear regression (β, 0.370, 95% CI, 0.062–0.320, p = .005) than those with normal troponin. However, elevated troponin was not associated with hematoma volume at admission and the change in hematoma volume from diagnostic to follow up CT. Figures 1 and 2 showed the variation of troponin along with hematoma volume at baseline and follow-up in the scatter plot and box diagram.
4 Discussion
Cerebral herniation is a rare and devastating complication in ICH patients. Intracerebral hematoma could lead to the rapid increase of cerebral pressure and result in the downward displacement of brain tissue through the notch (Wu et al., 2014). To date, the main reports of cerebral herniation consist mostly of clinical reports (Rehman, Ali, Tawil, & Yonas, 2008; Sampaio, Dias da Costa, Rocha, & Leao, 2016), and information about herniation after ICH reported in the literature is limited.
In this retrospective cohort study, we found that elevated troponin was not just associated with the frequency of cerebral herniation, but also large hematoma volume at follow-up, suggesting that serum troponin levels played a pivotal role in ICH patients and are associated with the severity of stroke. Furthermore, we observed that ICH patients with elevated troponin were more likely to have a higher systolic blood pressure levels, a higher frequency of CKD and ESRD and a more frequent deep location, possibly shared the pathophysiology of cerebral herniation. However, the change in hematoma volume from diagnostic to follow up CT between patients with and without elevated troponin, as well as between those with and without cerebral herniation, was not significant. Previous study reports that approximately one-third of ICH patients had significant change in hematoma volume (Brott et al., 1997), suggesting that not all patients had the risk of change in hematoma volume or hematoma expansion. The CT spot sign, hematoma morphologic appearance (Blacquiere et al., 2015), and the etiologic subtype (Cappellari et al., 2015) also had great influence on change in hematoma volume.
The mechanisms that can explain the association of elevated troponin with high frequency of brain herniation are complex and incompletely understood. First, Cardiac troponin is a highly sensitive and specific marker of myocardial injury (Agewall, Giannitsis, Jernberg, & Katus, 2011; Thygesen, Alpert, & White, 2007). The rapid increase of intracranial pressure, especially in patients with cerebral herniation, can induce the fast-releasing catecholamines as a result of sympathoadrenal activation which act on myocardial cell (Hamann et al., 1995; Leow, Loh, Kiat Kwek, & Ng, 2007). The high concentration of catecholamines in myocardial cell would cause a calcium abnormality (Mann, Kent, Parsons, & Cooper, 1992) and bring about a reduction of contractility in the myocardium. Second, elevated cardiac troponin was associated with large hematoma volume at follow-up in bivariate correlation and linear regression, which adjusted potential confounders such as age, GCS score and systolic blood pressure at admission, than those with normal troponin in our study. In addition, ICH patients with elevated troponin had a higher systolic blood pressure and diastolic blood pressure. To our knowledge, high blood pressure may promote on-going bleeding in brain and even caused hematoma expansion, and promoted the formation of encephaledema through hydrostatic or oncotic pressure gradients (Chen, Chen, Hsu, & Hogan, 1989; Sykora et al., 2008). Third, elevated troponin may reflect poor renal function. In the present study, elevated troponin was associated with high frequency of CKD and ESRD. The results were in accordance with recent studies about the relationship of troponin and renal function (Teo, 2015). Furthermore, renal dysfunction had a 2.3-fold higher hematoma volume compared to those with normal renal function (Molshatzki et al., 2011). And these findings indicated another mechanism for large hematoma volume and high frequency of cerebral herniation.
Our study indicated that ICH patients with elevated troponin had a higher proportion of in-hospital mortality. Whereas the relationship of elevated troponin to in-hospital mortality became no longer significant after adjusting confounding factors.
Limitations of this study include the single-center design and retrospective analysis with exclusion of those subjects without the data of serum troponin. Therefore, there might be selection bias in this study. Other limitations were small sample size for multivariable analysis, single measurement of troponin and the possible existence of the unmeasured confounding factors which may explain some of our findings. Future prospectively large studies are warranted to make this distinction.
5 Conclusions
Elevated troponin, even after adjusting potential confounders, is associated with an almost five-fold increased risk of cerebral herniation. The possibility of cerebral herniation should be considered when ICH patients with large hematoma volume and elevated troponin.
Acknowledgments
This study was supported by The National Key Research and Development Program of China, Ministry of Science and Technology of China (2016YFC1300500-505) and the National Key Technology R&D Program and the 12th Five-Year Plan of People's Republic of China (2011BAI08B05).
Conflicts of Interest
None declared.